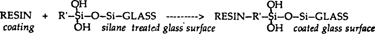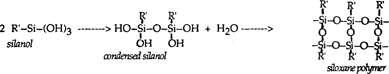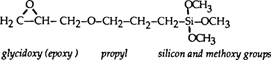Topics in Photographic Preservation 1989, Volume 3, Article 11 (pp. 69-85)
A Preliminary Study: Consolidation of Gelatin Glass Plate Negatives with Organosilanes
by Sarah S. Wagner*
Abstract
Organosilanes (silanes) are compounds containing silicon and organic functional groups. Their bifunctionality makes them ideal coupling agents for normally incompatible materials such as glass and organic resins. This study focuses on the use of one organosilane, 3-Glycidoxypropyltrimethoxy silane, for the consolidation of flaking gelatin emulsion on glass plate negatives. In the study, experimental emulsion samples were adhered to glass microscope slides using various treatment protocols. Results on experimental samples indicate that the silane was effective as a consolidant. Consolidation of actual glass plate negatives was achieved with limited success. The silane passed the ANSI IT9.2 Photo Activity Test. The use of any silane is not considered to be readily reversible.
Introduction
Deterioration of glass plate negatives
Glass plate negatives suffer from several types of deterioration:
- –glass breakage and mechanical damage to the emulsion,
- –glass deterioration,
- –emulsion deterioration and staining,
- –yellowing and embrittlement of any varnish layers, and
- –flaking or delamination of the emulsion.
Deterioration, combined with extreme environmental conditions, can contribute to the loss of adhesion between the emulsion and the glass support. The emulsion can lift off in sheets, shatter and crack, or frill at the edges of the plate. Lifting of the emulsion from the glass plate negative is a common problem and one not easily solved.
Loss of adhesion to glass supports
Adhesion of any material to glass is tenuous at best and can be disrupted easily. Adhesion of organic compounds to glass is by weak secondary bonds such as van der Waal's forces and dispersive (dipole) forces formed as the material wets out onto the glass surface (1). When the coating solidifies, the solid polymer may no longer wet the surface. This poorly adhered layer can fail easily at the glass interface (2). Moisture attacks the glass/coating interfacial bond by being adsorbed onto the glass surface and disrupting the weak secondary bonds of adhesion (3). In addition, dimensional change in the coating relative to the glass support creates stress on the interfacial bond. This stress can shear the weak bonds of adhesion and cause the emulsion to delaminate from the glass (4).
Susceptibilty of gelatin emulsions to deterioration
Gelatin is hygroscopic and therefore reactive to changes in relative humidity and temperature. How susceptible any one gelatin emulsion is to environmental conditions, and possible stress induced shearing from the glass support, depends on numerous and complex factors. These factors include:
- –the thickness of the emulsion layer,
- –temperature and relative humidity,
- –salt concentration within the emulsion,
- –the presence of deliquescent salts and glass decomposition products on the glass surface,
- –processing of the negative, and
- –the manufacture of the gelatin itself (5).
Since the factors applicable to glass plate negatives vary, they are not equally susceptible to flaking and cracking. In any one collection, some negatives will be stable, others might exhibit minor flaking, while some will suffer almost complete delamination. Seemingly stable negatives can develop flaking unexpectedly with age, or as storage conditions change (6).
Difficulty of conservation using traditional methods
The conservation of flaking gelatin emulsions on gelatin dry plates presents several problems and has defied a satisfactory solution. Duplication is an obvious preservation measure, but does not solve the inherent problems of the original object. In extreme cases, negatives can not be duplicated, handled, or even safely removed from their housings (6).
The use of adhesives to reattach the flaking emulsion to the glass support is also problematic for the following reasons:
- –Adhesive failure is likely because organic coatings do not bond well to inorganic surfaces, especially glass surfaces. Surface dirt and oils, and glass deterioration products commonly found on historic objects, interfere with the bonding and wetting of adhesives onto glass (2);
- –The gelatin emulsion is highly reactive to the presence of moisture. This makes the use of aqueous adhesives difficult. Gelatin, synthetic polymer emulsions, and cellulose ethers fall into this category;
- –Most adhesives, whether emulsion or solution type, adversely affect the reflectance and transmittance qualities of the negative and thereby interfere with printing or viewing (7). The adhesive may act as a varnish on the negative or it may be thickly applied in areas of lifting. This problem is even more severe if the lifted emulsion is distorted, because extra adhesive is required to fill the gaps and voids between the smooth glass and uneven surface of the emulsion film;
- –The viscosity needed for good adhesion interferes with the ability of the solution to flow into areas of blind cleavage or under areas of large flakes;
- –Many adhesives adversely affect the silver image/gelatin emulsion stability (8).
Ideally, an adhesive for the consolidation of glass plate negatives would be:
- –clear,
- –low viscosity,
- –wet easily onto both glass and organic surfaces,
- –bond well to glass,
- –be stable to degradation and moisture attack,
- –not adversely affect the silver gelatin emulsion, and
- –be reversible. Reversibility is desirable, but not necessarily a practical issue given the reactivity of gelatin to moisture and solvent treatment.
Organosilanes and their interaction with inorganic and organic materials
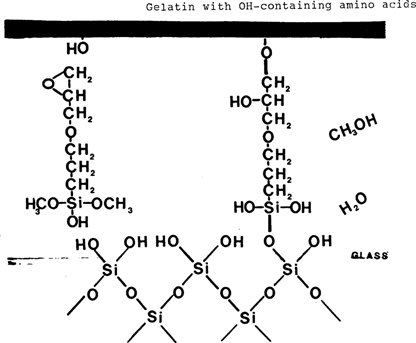
Organosilanes (silanes) fulfill many of the criteria for an ideal adhesive. Organosilanes are not adhesives in a technical sense, but rather act as coupling agents. That is, they are bifunctional compounds with the ability to covalently bind to both inorganic and organic materials. Silanes contain both a silicon group and an organic group in their structure, and have the generic formula R'–Si–(OR)3. The generic silane undergoes the following reactions when used as a coupling agent between a resin coating and a glass surface (4). Water is considered necessary for the first reaction step to occur. Water is considered present on the glass surface or in ambient humidity. An aqueous solution can be used, also.
1) Hydrolysis to form the reactive silanol

2) Covalent bonding of silanol group with OH groups on inorganic, silicon based material (glass)

3) Covalent bonding of silane organofunctional group with organic resin by reaction characteristic of the two
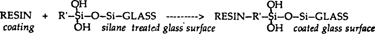
4) Self condensation and polymerization of silane
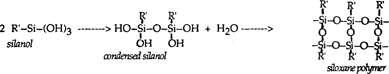
As seen in reaction 3 above, the organosilane forms a covalent, chemical “bridge” between the organic coating and glass. This bridge is usually several molecular layers thick and includes some polymerized siloxane polymer as shown in reaction 4 (4) (silane which has polymerized to a siloxane polymer is analogous to silicone rubber). The bonding is covalent, strong, stable to moisture and other degradative influences (10,11).
Uses of organosilanes
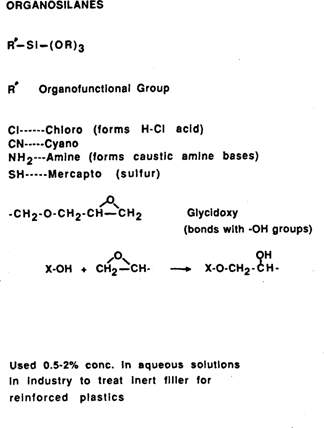
Organosilanes are widely used in industry for reinforced plastics containing inorganic fillers, coatings technology, and as waterproofing agents (4). Silanes, or mixtures of silanes and organic resins, have been used in conservation for the treatment of glass, stained glass, stone, and polychromed stone (2,12,13,14,15,16,17). A number of organosilanes exist, each having different organofunctional groups and/or alkoxy groups off of the silicon. In industrial applications, they are chosen for the characteristic reaction that the functional group undergoes with other organics and for proven compatibility and effectiveness (18). (Appendix 1)
Proposed use oforganosilane for consolidation of glass plate negatives
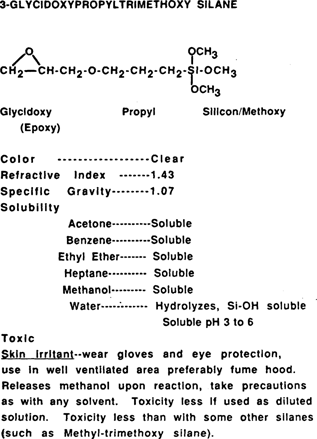
This study investigates one organosilane, 3-Glycidoxypropyltrimethoxy silane, for its effectiveness in the consolidation of gelatin glass plate negatives (refer to Appendix 2 for more chemical information). The formula for 3-Glycidoxypropyltrimethoxy is:
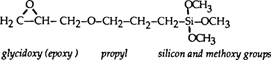
The organofunctional group on this silane is an epoxy. Epoxy groups readily undergo characteristic reactions with hydroxyls (OH) to open up the strained epoxy bonds, as follows (19).

3-Glycidoxypropyltrimethoxy silane was chosen because its epoxy functional group has the potential to react with OH groups present on amino acids found in the gelatin protein (20,21)(Appendix 3). There are several OH containing amino acids in gelatin. Hydroxyproline is the most common, making up about 10% of the gelatin protein. These amino acids and their OH groups tend to be oriented towards the outside of the helical gelatin protein strand thereby increasing their availability for reaction (22).
Experimental Procedure
This research project consisted of four parts:
- 1) The consolidation onto glass microscope slides of samples cut from a sheet of emulsion which had delaminated from a glass plate negative. Treatment conditions and silane solution concentrations were varied. Samples were evaluated for change in size (area), degree of adhesion, voids between emulsion and glass, surface characteristics and general visual appearance.
- 2) The consolidation of actual glass plate negatives with the method found to be most successful in Part 1. Evaluation same as above.
- 3) Testing for the reversibility of recently treated and seven month old samples with acetone, ethanol, and water.
- 4) Testing for the effect of the silane on image and gelatin stability using the Photo Activity Test ANSI IT9.2 (23). Evaluation based on visual appearance only (densitometry readings not taken).
Part One: Consolidation of experimental emulsion samples
Each sample set contained five samples of gelatin emulsion. Rectangular samples were cut from a large sheet of emulsion that had naturally delaminated from a glass plate negative. The samples were cut using a scalpel, straight edge, and 0.1 mil Mylar overlay while the emulsion sheet was under mild suction. The size of each sample was approximately 1×1.5 cm. Each sample was measured before and after treatment using an ocular reticle on an Olympus stereobinocular microscope (total magnification 63x). Length and width measurements were noted (ocular units only), and area calculated. Samples were placed on acetone cleaned glass microscope slides and treated as summarized below (treatments were done in a fume hood).
100% Silane, varied humidity sources
Set 10–100% Silane fed under sample and brushed on top, Parafilm placed on top for five days.
Set 6–Sample placed in 95% humidity chamber 15 minutes, 100% Silane fed under sample and brushed on top, Parafilm placed on top for five hours, sample left in tray one day.
Set 5–A stream of ultrasonic humidity allowed to flow over sample, 100% Silane fed under sample and brushed on top, Parafilm placed on top for five hours.
Set 4–100% Silane fed under sample and brushed on top, a stream of ultrasonic humidity allowed to flow over sample, Parafilm placed on top for five hours.
Set 2–95% Ethanol:5% Silane fed under sample and brushed on top, Parafilm placed on top for five days.
Ethanol:Silane mixtures, varied humidity sources
Set 8–95% Ethanol:5% Silane fed under sample and brushed on top, a stream of ultrasonic humidity allowed to flow over sample, sample placed in 95% humidity chamber one day, no Parafilm.
Set 11–90% Ethanol:10% Silane fed under sample and brushed on top, sample covered with thin Goretex and humid blotter and placed under 1/4 in. plexi for five hours.
Set 7–90% Ethanol:10% Silane fed under sample and brushed on top, sample placed in 95% humidity chamber one day, no Parafilm.
Ethanol:Silane:H2O mixtures, Parafilm, varied time periods
Set 3–90% Ethanol:5% Silane:5% H2O fed under sample and brushed on top, Parafilm placed on top for five days
Set 9–85% Ethanol:10% Silane:5% H2O fed under sample and brushed on top, sample placed in 95% humidity chamber one day, no Parafilm.
Set 18–90% Ethanol:5% H2O:5% Silane fed under sample and brushed on top, Parafilm placed on top for one day.
Set 12–95% Ethanol:5% Silane fed under sample and brushed on top, Parafilm placed on top for half a day.
Set 13–95% Ethanol:5% Silane fed under sample and brushed on top, Parafilm placed on top for one day.
Set 14–95% Ethanol:5% Silane fed under sample and brushed on top, Parafilm placed on top for three days.
Ethanol:Silane:H2O mixtures, Goretex, varied time periods
Set 15–95% Ethanol:5% Silane fed under sample and brushed on top, Goretex and 1/4 in. plexi placed on top for half a day.
Set 16–95% Ethanol:5% Silane fed under sample and brushed on top, Goretex and 1/4 in. plexi placed on top for one day.
Set 17–95% Ethanol:5% Silane fed under sample and brushed on top, Goretex and 1/4 in. plexi placed on top for three days.
The ambient relative humidity was 45% during the time that sets 1 through 11 were measured and treated. After treatment measurements were made three to seven days after the treatment was completed. A second set of after treatment measurements were taken three weeks after treatment for set 1 through 11, at which time the relative humidity had fallen to 40%. The ambient relative humidity was 40% during the time that sets 12 through 18 were measured and treated.
Part Two: Consolidation of actual glass plate negatives
Glass plate negatives with two types of flaking problems were treated:
- –One 8×10 negative suffered flaking characterized by a shattered, cracked emulsion over most of the negative.
- –Four 4×5 negatives suffered flaking characterized by the delamination of sheets of the emulsion.
The large negative and one small negatives were treated with 90% Ethanol:5% Silane:5% H2O.
One small negative was treated with 95% Ethanol:5% Silane. Two small negatives with contracted and distorted flaking sheets were treated with 85% Ethanol:5% Silane:10% H2O.
The solution was introduced to the surface of the emulsion with an eye-dropper and allowed to flow under and through the emulsion. Parafilm was placed on top of the surface. The Parafilm was squeezed so that air bubbles were expelled from between the emulsion and glass to insure contact between the two surfaces. The negatives were placed face down on a hard surface (Plexiglas) for five days. The first treatment was unsuccessful, so all negatives were retreated. Fresh Parafilm was placed on top, then blotter, 1/4 in. plexi and a small weight. The negatives were left for seven days before the Parafilm was peeled off. (Procedure done in a fume hood).
Part Three: Testing for reversibility in solvent baths
Treated samples that were one month old and seven months old were placed in beakers containing one of these solvents: acetone, ethanol, or water. Two seven month old samples and two one month old samples were treated with each solvent. Samples were removed at intervals of 1,5, and 8 hours, 1 and 5 days to test for adhesion of the emulsion to the glass slide.
Part Four: ANSI IT9.2 Photo Activity Test
100% silane and 90% Ethanol:10% Silane were tested for their effect on silver image stability and gelatin staining using ANSI IT9.2 Photo Activity Test. The fading detector (colloidal silver), staining detector (fixed-out gelatin photographic paper), and Whatman Chromatography Paper samples were prepared according to ANSI IT9.2 (the silane solution was applied directly to the detector), and aged for 15 days at 86% RH and and 70 C along with controls. Samples were evaluated visually. Densitometry measurements were not performed.
Results
Part One: Treatment of experimental emulsion samples
Results are summarized in Table 1. Most samples were adhered successfully to some degree or another. Excel 1.5 Spreadsheet Software on a Macintosh Computor was used to calculate Area (LxW), Average Area, % Change Area ± S. Percent Change Area is relative to the before treatment size and was calculated as AreaBT-AreaAT/AreaBT. Sample size is five for each set. Unit measurements were ocular units only and were not calibrated to a metric scale. Some sets were measured twice–the second set of measurements (*) was taken three weeks later. At that time, the relative humidity had fallen to 40% from the initial 45%.
Table 1 Treatment of experimental emulsion samples.
SET |
TREATMENT |
% CHANGE AREA |
COMMENTS |
1 |
Water |
+0.4±1.8 |
−1.6±2.2* |
Fail, no adhesion |
10 |
Silane, Parafilm 5d |
−9.4±0.8 |
−12.5±0.5* |
Good adhesion, voids, 2 week set time |
6 |
RH Tray, Silane, Parafilm 5 hr, RH Tray 1d |
+0.9±1.1 |
−0.4±0.9* |
Very good adhesion, tide line inside edge of sample |
5 |
Ultrasonic RH, Silane Parafilm 5 hr |
+2.7±1.6 |
+0.7±0.6* |
Fair adhesion, large voids |
4 |
Silane, Ultrasonic RH Parafilm 5 hr |
+1.9±0.9 |
+0.8±1.1* |
Fair adhesion, large voids |
2 |
95 ETOH:5 Silane, Parafilm 5d |
+3.8±1.8 |
+1.1±1.0* |
Very good adhesion |
8 |
95 ETOH:5 Silane Ultrasonic RH, RH Tray 1d No Parafilm |
−9.0±2.9 |
−10.8±3.9* |
Very good adhesion |
11 |
90 ETOH:10 Silane Goretex/Blotter 5 hr |
−7.9±2.2 |
−11.0±2.0* |
Fair adhesion, not uniform |
7 |
90 ETOH:10 Silane, RH Tray 1d, No Parafilm |
−8.1±2.7 |
−12.1±2.4* |
Fair adhesion, not uniform |
3 |
90 ETOH:5 Silane:5 H2O Parafilm 5d |
+4.9±1.4 |
+1.4±0.9* |
Good adhesion, small voids |
9 |
85 ETOH:10 Silane:5 H2O Parafilm 5d |
−8.1±1.5 |
−12.5±1.4* |
Very good adhesion |
18 |
90 ETOH:5 Silane:5 H2O Parafilm 1d |
−0.3±0.9 |
|
Fair to good adhesion, not uniform |
12 |
95 ETOH:5 Silane Parafilm 1/2 d |
+0.1±0.6 |
|
Fail, spotty adhesion |
13 |
95 ETOH:5 Silane Parafilm 1d |
−1.0±4.0 |
|
Fail, spotty adhesion |
14 |
95 ETOH:5 Silane Parafilm 3d |
−0.2±1.5 |
|
Fail, spotty adhesion |
15 |
95 ETOH:5 Silane Goretex 1/2 d |
−0.3±0.6 |
|
Fail, spotty adhesion |
16 |
95 ETOH:5 Silane Goretex 1d |
−1.8±3.9 |
|
Fail, spotty adhesion |
17 |
95 ETOH:5 Silane Goretex 3d |
−0.7±0.4 |
|
Fail, spotty adhesion |
Part Two: Consolidation of actual glass plate negatives
Refer to Table 2. Consolidation was unsuccessful on all plates after the first treatment, therefore all negatives were retreated. The 8×10 negative with shattered emulsion was adhered after the second treatment. The four 4×5 negatives with flaking sheets of emulsion were not adhered.
Table 2 Consolidation of actual glass plate negatives.
TREATMENT |
COMMENTS |
8×10 Negative |
|
90 Ethanol:5 Silane:5 H2O |
|
Parafilm 5–7 days |
Fail–most flakes did not adhere and peeled off with Parafilm |
Repeat treatment |
Good adhesion–few flakes peeled off with the Parafilm |
4×5 Negative |
|
95 Ethanol:5 Silane |
|
Parafilm 5–7 days |
Fail–no adhesion |
Repeat treatment |
Fail-no adhesion |
4×5 Negative |
|
90 Ethanol:5 Silane:5 H2O |
|
Parafilm 5–7 days |
Fail–no adhesion, some curling of emulsion |
Repeat treatment |
Fail-no adhesion, some curling of emulsion |
4×5 Negative |
|
90 Ethanol:5 Silane:5 H2O |
|
Parafilm 5–7 days |
Fail–no adhesion, some curling of emulsion |
Repeat with 95:5 solution |
Fail-no adhesion. Partial adhesion-placing under table lamp caused emulsion to contract and curl slightly, then lift from glass |
4×5 Negative |
|
85 Ethanol:5 Silane:10 H2O |
|
Parafilm 5–7 days |
Fail–no adhesion, severe curling of emulsion |
Repeat with 95:5 solution |
Fail-no adhesion, severe curling of emulsion |
Part Three: Testing of reversibility with solvent baths
Refer to Table 3. One one-month old sample released in acetone and water. Seven month old samples did not release in any solvent, except for one sample treated with water. All samples were distorted after treatment.
Table 3 Testing for reversibility with solvent baths.
SILANE TREATED SAMPLES NATURALLY AGED |
ACETONE |
ETHANOL RELEASE TIME |
WATER |
One month old |
8 hours |
5 days |
2 hours |
Seven month old |
adhered > 5 days |
adhered > 5 days |
8 hours adhered > 5 days |
Part Four: ANSI IT9.2 Photo Activity Test
Refer to Table 4. No silver fading or gelatin paper staining occurred. A very faint yellow tone developed on gelatin paper samples.
Table 4 ANSI IT9.2 Photo Activity Test.
SILANE SOLUTION |
FADING DETECTOR COLLOIDAL SILVER |
STAINING DETECTOR GELATIN PHOTO PAPER |
100% Silane |
No fading/mottling No staining/very faint yellow |
|
90%:10% Ethanol:Silane |
No fading/mottling |
No staining/very faint yellow |
Discussion
Consolidation of experimental emulsion samples
Treatment protocols were varied in Part One in order to ascertain if 3-Glycidoxypropyltrimethoxy silane could be used effectively under conditions appropriate for the safe treatment of actual glass plate negatives.
Treatment methods on sample sets fell into four types of categories:
- 1) Use of 100% Silane with varied humidity sources.
- 2) Use of Ethanol:Silane with varied humidity sources.
- 3) Use of Ethanol:Silane:H2O covered with Parafilm for varied time periods.
- 4) Use of Ethanol:Silane:H2O covered with Goretex for varied time periods.
Good to very good adhesion was obtained with most treatment protocols in all categories except category 4. However, the number of air pockets or voids between the glass and emulsion varied, as did the % Change in Area, depending on the treatment.
The presence of air pockets was more likely if the emulsion was distorted, because distortions create gaps between the emulsion and glass surface. A small amount of weight probably would have ensured better contact between the emulsion and glass. However, weight was not applied because the Parafilm appeared to keep the the emulsion in contact with the glass during the experiment.
The presence of numerous or large air pockets is considered unacceptable for two reasons:
- –adhesion is less uniform or complete and therefore more likely to fail;
- –air pockets are visible in transmitted light and would affect the quality of any duplicate negative or print.
In category 1 through 3, some treatment protocols resulted in a 8 or 9% decrease in size (sets 10,8,11,7,9) while other treatments resulted in a size increase of 1 to 5% (sets 6,5,4,2,3). Large decreases in size are unacceptable because delaminated emulsion on actual negatives usually is contracted to some degree–adjacent flakes no longer match up resulting in gaps between flakes. By the same reasoning, a small increase in size may be welcome as long as adjacent flakes do not swell so much as to overlap eachother.
General trends observed from experimental emulsion samples
Evaluation of experimental emulsion samples reveals several trends. Large relative decreases in area occurred mostly with those samples treated with high concentrations of silane (100%, 10%– sets 10,11,7,9) or prolonged exposure to moisture (95% humidity tray-sets 8,7; Goretex/humid blotter-set 11). Small relative increases in area occurred with short exposure to humidity (ultrasonic humidity-set 4,5) or small concentrations of silane and water in ethanol solutions (95% Ethanol:5% Silane-set 2; 95% Ethanol:5% Silane:5% H2O-set 3).
The unexpectedly large relative decrease in area found in treatments with high concentrations of silane and humidity is similar to results obtained in stone consolidation experiments (9). In a study investigating the effect of relative humidity on the consolidation of stone with Methyltrimethoxy Silane, high humidity resulted in rapid setting times and stress induced cracks. Presumably, rapid setting (polymerization by self condensation) within the stone block caused the silane to contract so quickly that stress could not be dissipated. As a result, cracks developed in the stone block.
It seems likely that a similar mechanism was involved in the treatment of samples in this study, except that shrinkage was not restrained by the emulsion. Instead, the whole emulsion contracted along with the polymerizing silane. Less shrinkage occurred when the humidity was limited (as with ultrasonic humidity exposure) because the reaction did not occur as quickly. Less shrinkage occurred with small concentrations of silane, because there was not enough silane available to polymerize, that is self condense.
The amount of shrinkage increased as the relative humidity levels dropped from 45% to 40%. This is probably due to the fact that the emulsion contracted as ambient moisture levels decreased. Fortunately, adhesion has not been affected by the natural response of the gelatin to its environment. It appears that the silane induced adhesion has some resilience; that is, the silane-glass bond can accomodate small stresses created by the natural expansion and contraction of the gelatin in response to the changes in relative humidity. Such bond resilience is supported in the literature on silanes (4).
Best results for experimental samples
Best results were obtained with three treatment protocols:
- Relaxation in a 95% Humidity Tray, 100% Silane application, Parafilm cover for five hours, then continued exposure to 95% humidity for one day (set 6).
- Treatment with 95% Ethanol:5% Silane, Parafilm cover for five days (set 2).
- Treatment with 90% Ethanol:5% Silane:5% H2O, Parafilm cover for five days (set 3).
Superior relaxation of distortions and very good adhesion was achieved with the use of the humidity tray. However, tiny air bubbles developed aound the inside margins of the sample. The presence of these air bubbles was considered a disadvantage for duplication or printing purposes. Good adhesion was obtained with ethanol and silane mixtures, although slightly more relaxation of distortions and a small size increase was obtained when water was added to the mixture.
Parafilm as a cover seemed effective. It was flexible, inert, smooth (but not slick), and could be peeled back easily. Poor adhesion occurred with the use of the Goretex cover.
Treatment of actual glass plate negatives
Actual glass plate negatives were treated with 95% Ethanol:5% Silane and 90% Ethanol:5% Silane:5% H2O because of the favorable results obtained with the use of these mixtures in Part One of the study. The amount of water was increased to 10% for the treatment of two negatives with severely distorted and contracted emulsions in the hopes that additional water would aid in relaxation and expansion. The addition of extra water did increase the relaxation and realignment of delaminated emulsion, but the treatment was unsuccessful and resulted in severe curling of the emulsion. In fact, the treatment of actual objects was unsuccessful, except in the case of the 8×10 negative with a fractured emulsion (95% Ethanol:5% Silane:5% H2O mixture).
Reasons for poor results obtained with actual objects
There are several reasons why better adhesion was obtained with experimental samples than with actual glass plate negatives. The use of emulsion samples/glass microscope slides as an experimental system provides conditions that are most favorable for silane induced adhesion:
- –relatively clean, stable (undeteriorated) glass, and
- –small undistorted emulsions that can be kept in uniform contact with the glass surface.
Theoretically, the silane should work as well on actual objects as it did on experimantal samples. However, two factors reduce its efficacy for the consolidation of actual glass plate negatives:
- –grime, oils, salts, and glass decomposition products on the emulsion and glass; and the fact that
- –silanes are not gap-filling adhesives and do not have adhesive properties per se.
Surface contamination interferes with the reaction of the silane to both the glass and gelatin by
- –creating a surface pH unfavorable for silane-glass bonding (3),
- –blocking potential reaction sites, and
- –reducing the amount of direct contact possible between emulsion, glass, and silane.
In some cases, adhesion may be improved if large flaking sheets of emulsion can be lifted and the glass surface cleaned by wiping with solvent soaked swabs. For obvious reasons, this procedure is not always possible.
Distortions in the emulsion create gaps between the two surfaces, resulting in air pockets and discontinuous adhesion. Because silanes are not gap filling adhesives, adhesion may be improved if the emulsion can be relaxed by judicious use of moisture and held in contact with the glass by weighting. Weighting unrelaxed emulsion does not seem to increase adhesion due to the ‘memory’ of the gelatin–once unweighted the emulsion springs back to its original shape and lifts off of the glass surface.
The tendency of the emulsion to return to a distorted state creates stress on the emulsion/silane/glass interfacial bond. This stress can break the silane bonds, especially if moisture is present (4). Although the silane bond to glass or to the coating is stable and covalent, it is not impervious to stress and moisture. Excessive stress can cause the bonds to shear, while the presence of moisture eventually hydrolyzes the silane/glass bond. In the case of 3-Glycidoxy-propyltrimethoxy silane, the bond to the organic coating is considered mildly susceptible to hydrolysis (4). But it should be noted that industrial testing for susceptibility to moisture is determined by how long a sample can remain immersed in boiling water without adhesion failure!
Potential for failure with silane consolidation
It is important to keep in mind that silanes were not intended to be used as coupling agents between glass and a resin coating which has already solidified. In industrial applications, silanes are used in three basic ways:
- –in dilute, slightly acidic aqueous solutions (0.5–2.0%, pH 4–7) as a pretreatment ‘surface primer’ for inorganic fillers such as glass fibers (for fiberglass);
- –or the silane is mixed directly with liquid resin solutions containing inorganic fillers such as metal filings or mineral particles (the silane migrates to the filler-resin interface as the mixture sets)(13,4).
- –In some instances silanes are used in combination with a compatible adhesive in order to adhere two solid, inorganic surfaces (such as metal or glass).
When evaluating results from a procedure that greatly modifies the optimal conditions for a product's use, one must be aware of the likely pitfalls. Only then can non-optimal conditions be optimized within the narrow confines demanded by sound conservation treatments.
Conclusions
Conclusions about the use of silanes for consolidation of glass plate negatives
Despite these drawbacks, results of this study are encouraging and warrant the continued study of 3-Glycidoxypropyltrimethoxy silane as a consolidant for glass plate negatives. The results from the treatment of experimental samples indicate that 3-Glycidoxypropyltrimethoxy silane can be used effectively under a number of treatment protocols. More experimentation on actual objects and experimental samples is needed in order to refine the treatment process and determine if silane consolidation is possible on real glass plate negatives. An alternative approach would be to investigate the use of silanes in cases where emulsions have been stripped from their glass supports and transferred to new glass (24,25,26).
The use of a humidity chamber for relaxation should be tried on the ethanol/silane, ethanol/silane/water mixtures, and with 100% silane. The use of a humidity chamber and 100% silane was successful on experimental samples, but was not tried on actual objects in this study. It may hold more promise, because the larger amount of silane can self condense to form a more resinous layer of siloxane polymer (analogous to silicone rubber). This polymer mass, though thin, may have more of the qualities of traditional gap filling adhesives.
One disadvantage of the use of silanes is the fact that they are not reversed easily once adhesion has occurred. Silane residues can be removed from the surface of treated emulsions with ethanol soaked swabs without loss of adhesion within limited time periods. Tests done in this study indicate that emulsions adhered to glass with 3-Glycidoxypropyltrimethoxy silane can be reversed in acetone and water at least one month after treatment. However, the use of solvents or water can have disasterous effects on the emulsion, such as shrinkage and distortion. Of course, the same can be said of solvents or moisture applied to reverse traditional adhesives used to re-adhere emulsion to glass supports.
One reason for continued research into the use of silanes for glass plate negative consolidation is the favorable results of the ANSI IT9.2 Photo Activity Test. 3-Glycidoxypropyltrimethoxy silane did not adversely affect the silver image or emulsion stability in regards to fading or staining when tested. The same can not be said for many adhesives commonly used in conservation. However, long term aging characteristics of this silane are not known. Long term aging properties should be investigated, along with repeated testing with the Photo Activity Test in order to statistically verify that 3-Glycidoxypropyltrimethoxy silane is not unstable, likely to discolor, or harm photographic materials.
Acknowledgments
This study could not have been performed without the encouragement and support of two institutions and several people. Connie McCabe, Photograph Conservator at the National Archives and Records Administration provided guidance, technical advice, encouragement, and help with the evaluation of the results. In addition, Connie performed the Photo Activity Test. NARA graciously allowed Connie the time to help me with this project. Doris Hamburg and Sylvia Albro of the Conservation Office/Library of Congress were very supportive of my research and provided materials and encouragement, while Peter Waters showed me how to use Excel 1.5 Spreadsheet Software with good humor and patience. Lastly, I should thank Debbie Hess Norris for convincing me to do this project.
Materials
3-Glycidoxypropyltrimethoxy Silane (Fluka Chemical Corp. Ronkonoma, NY)
Ethanol
Distilled Water
Olympus Stereobinocular Microscope with 10x ocular reticle
Fisher Brand Precleaned, Frosted End, Glass Microscope Slides, 3 in.x1 in.
Sunbeam Ultrasonic Coolspray Humidifier with Nalgene Tubing Attachment
Parafilm, American Can Corp.
Winsor and Newton 00 Sable Brushes
Fisher Brand 25 ml Glass Stoppered Glass Bottles
Photo Activity Test (Image Permanence Institute, Rochester, NY)
References
(1) Holland, L. The Properties of Glass Surfaces, Chapman and Hall, London, 1966
(2) Errett, R.; Merrill, L.F.; Brill, R.H. “The Use of Silanes in Glass Conservation”, Adhesives and Consolidants IIC Preprints, 1984, 185–190.
(3) Eckstein, Y.; Berger, E. “The Effect of Silane on Glass/Resin Adhesion”, In Adhesive Chemistry–Developments and Trends Lieng-Huang Lee (Ed.); Plenum Press, New York, 1984, 139–163.
(4) Plueddemann, E.P. Silane Coupling Agents, Plenum Press, New York, 1982.
(5) Hendricks, K.B.; et.al. “Properties and Stability of Gelatin Layers in Photographic Materials”, AIC Preprints, 1984, 52–62.
(6) Personal Communication, Connie McCabe, Photograph Conservator, National Archives and Records Administration, Washington, DC, Winter 1989.
(7) Personal Communication, Debbie Hess Norris, Professor of Photograph Conservation/Ass't. Director, University of Delaware/Winterthur Art Conservation Program, Winter 1989.
(8) Personal Communication, Kim Schenk, Ass't. Paper Conservator, Baltimore Museum of Art, work to be published as “Preliminary Testing of Adhesives Used in Photograph Conservation”, Topics in Photographic Conservation, vol.3, 1989.
(9) Charola, A.E.; Wheeler, G.E.; Freund, G.G. “The Influence of Relative Humidity in the Polymerization of Methyl Trimethoxy Silane”, Adhesives and Consolidants IIC Preprints, 1984, 177–181.
(10) Bisconti, G.; et.al. “Stability Study of Siliconic Resins Employed in Stone Conservation”, ICOM Preprints, 8th Triennial Meeting, Australia, 1987, 785–790.
(11) Charola, A.E.; Tucci, A.; Koestler, R.J. “On the Reversibilty of Treatments with Acrylic/Silicone Resin Mixture”, JAIC, 1986, 83–92.
(12) Larson, J.; Dinsmore, J. “The Treatment of Polychrome Medieval English Sculpture in the Museum Environment”, Adhesives and Consolidants IIC Preprints, 1984, 167–170.
(13) Weintraub, S.; Greenland, M. “Field Application of Stained Glass Conservation Techniques”, Adhesives and Consolidants IIC Preprints, 1984, 199–201.
(14) Nishiura, T. “Laboratory Evaluation of the Mixture of Silane and Organic Resins as Consolidants of Granularly Decayed Stone”, ICOM Preprints 8th Triennial Meeting, Australia, 1987, 805–807.
(15) Hanna, S.B. “The Use of Organo-Silanes for the Treatment of Limestone in an Advanced State of Deterioration”, Adhesives and Consolidants IIC Preprints, 1984, 171–176.
(16) Horie, C.V. Materials for Conservation, Buttersworth, London, 1987.
(17) Grissom, C.A.; Weiss, N.R. “Alkoxysilanes in the Conservation of Art and Architecture” AATA Supplement, vol. 18, No.1, 1981, 150–199.
(18) Selection Guide to Dow Corning Organosilane Chemical, Dow Corning Corp., Midland, MI, 1985.
(19) Morrison, R.; Boyd, R. Organic Chemistry, Allyn and Bacon Inc., Boston, 1973.
(20) Seidler, R. The Use of Silanes for the Consolidation of Paintings on Glass, Unpublished Research, Student Science Project at the University of Delaware/Winterthur Art Conservation Program, 1987.
(21) Personal Communication, Richard Wolbers, Assoc. Professor of Paintings Conservation and Science, University of Delaware/Winterthur Art Conservation Program, Newark, DE 1988.
(22) Lehninger, A. Biochemistry, 2nd Edition, Worth Publishers, New York, 1977.
(23) Reilly, J.M.; Nishimura, D.; Pavao, L.; Adelstein, P.Z.; Photo Enclosures Research and Specifications, ANSI IT9.2, Image Permanence Institute, Rochester, NY, 1988.
(24) Personal communication, Doug Munson, Chicago Albumen Works, Housatonic, MA., March 1989.
(25) Personal communication, M. Susan Barger, PhD., Dept. of Materials Science and Engineering, Johns Hopkins University, Baltimore, MD, March 1989.
(26) Personal comunication, Martine Gillet, PhD., Centre de Recherches sur la Conservation des Documents Graphiques, C.N.R.S., Paris, France, March 1989.
Appendix 1
Appendix 2
Appendix 3
* Conservation Fellow, University of Delaware/Winterthur Art Conservation Program Conservation Intern, Paper Conservation Office, The Library of Congress, Washington, DC