Topics in Photographic Preservation 1991, Volume 4, Article 2 (pp. 14-30)
This study focused on the properties of four commercially available dry mount tissues (sometimes referred to as heat-set tissues) which are commonly used with photographs. The adhesives present were identified with Fourier transform infra-red spectroscopy, and their peel strength and color were evaluated on new and thermally aged samples. The suitability of their use with photographic materials was evaluated with a photographic activity test.
Almost since the inception of the photographic process in 1839, photographs have been adhered to secondary supports for display or for ease of handling. Early photographers understood that the materials that they chose for this purpose could effect their images and were therefore interested in their chemical properties. (1) Early adhesives were frequently water soluble, and included gums, starches, and proteinaceous materials. (2)
Dry mount tissues are generally, but not always, made of thin sheets of paper which have been coated on both sides with a thermoplastic adhesive. They were developed in order to provide a quick and easy method for adhering images overall to secondary supports. The process involves activating the adhesive with heat and bonding the two surfaces with pressure, thereby eliminating the need for water or solvents, presumably a decided advantage for many users. The use of dry mount tissues has been adopted by professional and amateur photographers, and by the custodians of large, archival collections (3), and their use is currently recommended in various publications. (4) Dry mount tissues are therefore frequently encountered on photographs, but conservators appear to know little about their components or properties. It is hoped that the simple tests used in this investigation will provide some basic information about the composition and aging properties of commercially available dry mount tissues, and about their possible effects on photographic materials.
Despite the frequency with which dry mount tissues have been used, there is little conservation literature which deals specifically with this topic.
In 1984, Lyons addressed the question of possible interactions of dry mount tissues with photographic materials. (5) Eleven dry mount tissues were placed in contact with photographic gray scales, and it was reported that no deleterious effects were seen. Unfortunately, the research has been published only as an abstract and the author has apparently left the field of conservation; therefore his experimental procedures are not known. A comparison of dry mount tissue resin formulas by Buchberg is also unpublished.(6)
In 1989, Schenck and McCabe conducted a preliminary investigation of adhesives used in photographic conservation using a procedure outlined in ANSI IT9.2–1988 (also known as the Photographic Activity Test).(7) The authors included in their investigation the commercially available dry mount tissue, Seal MT 5, and concluded that the dry mount tissue may be damaging to photographic materials.(8)
The majority of art conservation literature refers to problems associated with the removal of dry mount tissue from photographs. There seems to be consensus that some of these materials are not easily reversed, with the result that many conservators resort to immersing dry mounted photographs in solvent baths in order to remove the dry mount tissue.(5) This is a concern because the effects of organic solvents, or even water, on photographic binders have not been well researched.
Other concerns are the effects on photographic materials of the heat and pressure required for adhesion, and the possibility of damage during mounting (especially to resin coated papers, whose plastic coatings are easily melted). Conservators may also question the possible chemical interactions of dry mount tissues with photographic materials, although there is apparently some feeling that dry mount tissue may afford some protection for the photograph from poor quality secondary supports.(9,10)
Because of the scarcity of information which deals specifically with dry mount tissues, conservation literature dealing with adhesives and adhesives testing was also investigated. Landrock's Adhesives Testing Handbook (11) is an excellent general reference in the practical and technical aspects of the subject. An article by Allen (12) provides a summary of the various available primary and secondary forces of attraction and the types of bonds which eventually form. It also contains information regarding theories of adhesion and adhesive types.
The Canadian Conservation Institute (CCI) has an active adhesives testing program. Down (13) has identified the properties considered by CCI to be important in selecting an adhesive as well as methods for testing those properties. The author also discusses the problems with present testing procedures, primarily the apparently poor correlation that can sometimes exist between the results of artificially and naturally aged samples. (This is also discussed by Schenck and McCabe.)(8)
Also useful for the purposes of this investigation is a discussion by Bradley (14) of five adhesive strength test procedures developed by the American Society for Testing and Materials (ASTM), one of which has been adapted for use in this study (ASTM D 903–49). (15)
Because of the frequency with which dry mount tissues are used and the lack of conservation literature related to the topic, there is clearly a need for more information on the subject. The first goal of this study was to identify the adhesives used in commercially available dry mount tissues. It was also hoped that, once the materials were identified, their aging properties might be better understood, but this is highly speculative, as proprietary formulas are likely to contain additives which may not be fully identified by FTIR. Because of the feeling that dry mount tissues form strong bonds with photographs, a peel strength test was used to evaluate the strength of adhesion before and after accelerated aging, with the goal of confirming or denying this assumption, which appears to be based largely on observation. Yellowing was also evaluated with a densitometer before and after accelerated aging. Discoloration can be indicative of instability or degradation; furthermore, the color of a dry mount tissue may be important if that material is used with thin or translucent supports. Finally, because of the inherent sensitivity of many photographic materials, the dry mount tissues were also tested for deleterious effects on photographic binders and on silver final image materials, using a photographic activity test (PAT) developed by the American National Standards Institute (ANSI IT9.2–1988). (7)
Most of the samples used in the following experiments were obtained from their manufacturer in December 1989 (Seal Products, Naugatuck, Connecticut). However, some of the MT 5 used was obtained from Seal at an earlier date, and other examples of new MT 5 were obtained from the University Gallery at the University of Delaware. It was hoped that this would provide a better selection of replicates for this material, but it may have resulted in wide variability in the data generated within groups of MT 5 replicates, possibly as a result of a slight difference in proprietary formula or changes in physical and chemical properties as a result of aging that occurred before the samples were tested.
These dry mount tissues were chosen because they are readily available from either archival suppliers or camera supply stores, and because they could be obtained at no cost. It is understood that testing samples which are all manufactured by the same company may result in data that is not representative of all commercially available dry mount tissues; however, it is hoped that the data reflect those materials commonly used with photographs, as well as indicating some of the more subtle differences within a group of similar materials produced by the same manufacturer. It is also hoped that this investigation will help to evaluate the suitability of these test methods with commercially available dry mount tissues and similar materials.
While it is recognized that the results of accelerated aging may not correlate well with the results of natural aging, the samples in this investigation were subjected to high levels of heat and humidity in order to simulate the effects of aging. This practice appears to be a convention in conservation research and was necessary in order to complete this project within its allotted time frame.
The following dry mount tissues were used in this investigation, and their specifications, as identified by their manufacturer (1989), are also described: [Note that removability is defined by Seal Products by heat, not solubility.]
1) Archivalmount plus- an acid-free tissue which has been buffered to 7.5 plus pH with an alkaline agent and coated on both sides with an acid free adhesive, recommended temperature is 170 F (77 C), minimum temperature is 160 F (71 C), recommended for photographs and other materials, bond is considered removable with heat.
2) Colormount- a porous tissue (initial pH 6.9) coated on both sides with a low temperature adhesive (initial pH 7.0), recommended temperature is 200 F (93 C), minimum temperature is 180 F (82 C), recommended for resin-coated photographs and for general purpose mounting, the bond is considered permanent.
3) Fusion 4000 plus- an acid-free adhesive film (initial pH 7.0) with no paper base, recommended temperature is 190 F (88 C), minimum temperature is 160 F (71 C), recommended for general mounting purposes and textured surfaces, the bond is considered removable with heat. [Although Seal Products does not recommend the use of Fusion 4000 plus with photographs, it was included in this investigation because, apparently, this material is frequently used with photographs.]
4) MT 5- a glassine paper (initial pH 6.9) coated on both sides with adhesive (initial pH 7.0), recommended temperature 225 F (107 C), minimum temperature 185 F (85 C), recommended for fiber based photographs and other smooth porous materials, not recommended for non-porous materials or in high humidity conditions, the bond is considered permanent.
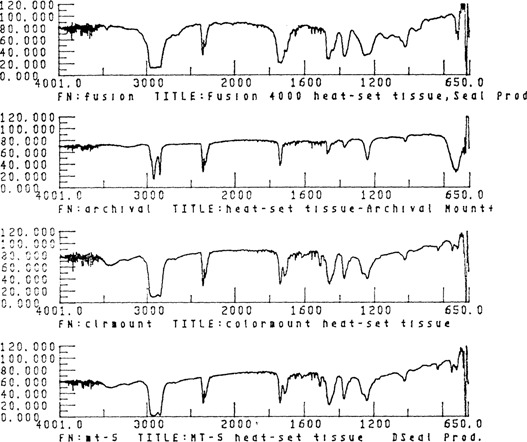
Samples of adhesives taken from the dry mount tissues were analyzed by Janice H. Carlson, Museum Scientist, Winterthur Museum, using an Analect RFX-65 Fourier Transform Infra-red spectrometer (FTIR) outfitted with a XAD microscope and an ATC-652 data handling system, located in the Scientific Research Laboratory of the Henry Francis duPont Winterthur Museum. The samples were prepared by scraping a tiny piece of adhesive from each of the dry mount tissues and placing the adhesive on a micro-KCL plate for FTIR microanalysis. The spectra generated were compared to spectra found in general infra-red spectroscopy reference manuals and to the information available in the instrument's computer (the polymer, art materials and reagent libraries were searched for the ten best matches). Although the spectra did not always match the references exactiy, presumably due to sample impurities and additives, accurate identification was possible.
This test for silver photographic images consisted of incubating the dry mount tissue against the surfaces of two detectors; one for fade, one for stain. The fade detector (obtained from the Image Permanence Institute, Rochester, NY) used was unprocessed colloidal silver in gelatin on a polyester base. The stain detector (manufactured at the National Archives) was a conventional, non-resin coated black and white photographic paper processed to minimum density according to manufacturers' instructions with a hypo clearing agent. The test was carried out in the Research and Testing Laboratory of the National Archives, under the supervision of Constance McCabe, Photographic Conservator.
The detector strips were measured for blue diffuse density in four randomly chosen locations, both before and after incubation. The readings were made using the blue status A filters of a MacBeth Transmission Reflection Densitometer, model TR-924, located in the Photographic Conservation Laboratory at Old College. A polyester template was used to identify the locations of the readings so that values obtained before and after aging would be taken from the same location. Transmission density was determined on the colloidal silver detectors, and reflection density on the photographic paper stain detector.
For each test, two “sandwiches” were made: a fade testing sandwich and a stain testing sandwich, each containing four replicates of a given dry mount tissue. Two similar sandwiches were made for controls using Whatman Number 1 filter paper. The same control was used for comparison with all of the dry mount tissues tested. Dry mount tissue samples and filter paper samples were cut to the same size as the detectors.
Four replicates were used for each set of tests and the detectors were randomly assigned to each replicate.
The fade testing sandwiches were made with four strips of fade detector, four strips of dry mount tissue (or filter paper), five pieces of uncoated mylar, and two pieces of glass. The stack was constructed so that the emulsion side of each detector faced a strip of dry mount tissue. The order was: glass, uncoated mylar, face detector, dry mount tissue, uncoated mylar, fade detector, dry mount tissue, uncoated mylar, fade detector, dry mount tissue, uncoated mylar, fade detector, dry mount tissue, uncoated mylar, and glass. This order deviates from that outlined in ANSI IT9.2 1988, and was chosen in order to place more replicates in each sample holder, thereby improving the statistical validity for the purposes of this investigation.
The stain testing sandwiches were made with four strips of stain detector, four strips of dry mount tissue (or filter paper), five strips of uncoated mylar, and two pieces of glass. The stack was constructed so that the emulsion side of each stain detector faced a strip of dry mount tissue. The order was: glass, uncoated mylar, stain detector, dry mount tissue, uncoated mylar, stain detector, dry mount tissue, uncoated mylar, stain detector, dry mount tissue, uncoated mylar, stain detector, dry mount tissue, uncoated mylar, and glass. This order also deviates from that outlined in ANSI IT9.2 1988, for the reason discussed above.
The dry mount tissues and standards in the sandwiches were under a pressure of 5 grams per square centimeter, which was adjusted by adding stainless steel weights to the surface.
These sandwiches were subjected to accelerated aging tests at 158 F (70 C) +/- 1 C and 86% +/- 2% relative humidity for 15 days. These conditions were obtained by storing the samples in a desiccator jar that was placed in a forced air circulating oven at 158 F (70 C). The 86% RH was obtained by keeping a saturated solution of barium chloride in water at the bottom of the jar.
The sandwiches were to be pulled apart immediately after they were removed from the desiccator jar in order to prevent adhesion between detectors and dry mount tissues. However, although the 158 F (70 C) temperature was above the activation temperature of the dry mount tissues, the adhesives apparently softened enough to adhere them to the detectors. The tissues were removed from the detectors by heating them through mylar with a tacking iron, and residual adhesive was removed with xylene. Ideally, these steps should be avoided because colloidal silver is very sensitive to heat and pressure, but, if left intact, the dry mount tissue and residual adhesive would have interfered with subsequent densitometry readings. It appears that the gelatin component of the stain detectors used with two of the MT 5 samples was softened and redistributed by the heat used at this point to activate the adhesive.
The incubated samples were evaluated in three ways: visually, by fade measurement, and by stain measurement.
The colloidal silver fade detectors were evaluated by transmitted light, using a light table, for the presence of mottling, which is defined by Webster's Unabridged Dictionary as “blotches or spots of different colors or shades of colors”. The presence of mottling is attributed to chemical interaction(s) between the colloidal silver fade detector and the dry mount tissue, and will cause failure of the PAT as defined by ANSI IT9.2 1988.
Stain and fade were evaluated by subtracting the initial blue density from the final blue density for each of the same four locations on the stain detector strips. The mean was calculated for the 16 density changes identified for each type of dry mount tissue (4 per each of four replicates). According to ANSI IT9.2–1988, a sample must fail the fade portion of the test (colloidal silver detector) if the mean density change is greater than the filter paper control fade value, defined as the mean of the control plus 2 X the standard deviation of the control. A sample must fail the stain portion of the test (gelatin detector) if the mean density change is greater than the filter paper control stain value, defined as the mean of the control plus 0.05. All calculations were made in densitometry units, and the instrument is accurate to within +/- .02 densitometric unit.
The peel strength test for adhesive bonds is designed to test the peel characteristics of adhesives on standard sized specimens. In this investigation, peel strength was evaluated both before and after various stages of accelerated aging.
For each combination of dry mount tissue and aging protocol, four replicates were made. Samples were composed of dry mount tissue, a rigid material (4-ply acid-free matboard), and a flexible material, Nomex (a synthetic aromatic polyamide polymer manufactured by DuPont Co.).
Initially it was hoped that the construction of the samples would closely mimic that of a typical photographic mount, and cellulosic papers were investigated as a material for the flexible member. Mock-up samples were made with photographic and other papers, and it was clear that these materials could not withstand the 180-degree turnback angle required for the test. Experimental samples were made with other materials, such as synthetic fiber webs and weaves, as well as Nomex. The woven materials were eliminated because it was proposed that their weave construction might provide a very different surface for mechanical bonding than that of a typical photographic paper. Experimental samples made from polyester were empirically proven to have very poor adhesion with the dry mount tissues to be tested, and were therefore eliminated. Nomex was ultimately chosen because it is a web construction, because preliminary tests indicated that its adhesion with the dry mount tissues would be measurable but not so great as to cause sample failure, and because it has excellent chemical, mechanical, and dimensional stability, even at high temperature and relative humidity conditions. It is understood that the bonding which will occur between the dry mount tissue and a polyamide is likely to differ from the bonding between a dry mount tissue and a cellulosic photographic paper, and that this test is not likely to simulate the behavior of a typical photographic mount: however, it is hoped that this peel strength test will provide some information about the aging properties of dry mount tissues.
Each sample consisted of a 1″ X 10″ piece of Nomex bonded for 6″ with dry mount tissue at one end to a 1″ X 7″ piece of matboard. (ASTM D 903–49 requires a flexible member of 1″ X 12″ and a rigid member of 1″ X 8″; however, the samples size had to be modified for this investigation due to the size of the sheets of Nomex provided by DuPont, and because of the 7 1/2″ diameter of the desiccator jar that the samples would eventually be aged in.)
The samples were prepared from one large piece of matboard from which 20 constructions of each dry mount tissue samples were eventually made. The matboard was divided into 16 pieces, measuring 7″ X 5″. Each piece was used to adhere one of the four replicates of each of the four dry mount tissues to the Nomex. The Nomex and matboard were predried, according to the Seal Products' recommendation, between Kraft paper in the Seal dry mount press located at the University Gallery in Old College. The temperature used for predrying was approximately 200 F (93 C), and the materials were predried under pressure, first for 45 seconds, then for an additional 30 seconds. The pressure is provided when the dry mount press is completely closed, and is not variable, and has therefore been assumed to have been consistently applied to all of the samples. Each of the dry mount tissues were placed between the matboard and the Nomex, and locally tacked in place with a tacking iron, according to the dry mount tissue manufacturers instructions. This may be suspected to create weaker or stronger adhesion in the tacked areas, but this step is necessary to insure proper alignment of all components. The samples made with Archivalmount plus, Colormount, and Fusion 4000 plus were sealed for 60 seconds at approximately 200 F (93 C), and the MT 5 was seal for the same amount of time at approximately 230 F (110). When the adhesives had cooled completely, the samples were cut into 1″ strips. The samples were then randomly assigned to one of five aging protocols.
Once the samples were aged, matboard fittings were adhered with Elmer's Glue-all to their tops and bottoms so that they would fit properly into the tensile test machine's clamps.
(ASTM D 903–49 requires that all specimens be conditioned for 7 days by exposure to 50% +/-2% RH at 73 F (23 C) +/- 2 C. This step was eliminated, because most of the samples were to be thermally aged, and those that were not were stored at approximately those conditions in the Photographic Conservation laboratory of Old College until such time as all of the samples could be evaluated for peel strength.)
Those samples which were randomly assigned to the accelerated aging protocols were artificially aged at 147 F (64 C) +/- 1 C and 86% +/- 2% relative humidity for 7 days, 14 days, 21 days, and 28 days in a sealed desiccator in a forced air oven. A potassium nitrate saturated salt solution was placed at the bottom of the desiccator in order to maintain the constant humidity. The samples were randomly placed in the jar, which was crowded. Each week, when the jar was opened, the samples were shuffled in order to alter their arrangement in the jar. Following artificial aging, all of the samples were stored for another 12 days in the Photographic Conservation Laboratory at Old College.
The peel strength was tested using an Instron Tensile Test Machine, model TTCML, which provided a separation of 5 cm/ min. (ASTM requires 6 in/ min, but this machine did not have the proper gear ratio for that rate.) The apparatus is located in the Mechanical Engineering facility of Spencer Laboratory at the University of Delaware, and its use was supervised by Ralf Tschirschnitz, Laboratory Coordinator. A 50 kg. maximum load cell was used to evaluate the samples. A smaller load scale would have provided a more precise peel strength reading; however, the size and shape of the samples would have required that special fittings for the machine be made, and it was beyond the budget of this project to provide them.
The flexible member of each sample was separated, when possible, from the rigid member by hand for a distance of about 1 in. The sample was then placed into the grips of the calibrated instrument, which was then adjusted to pull the flexible member back at a 180-degree angle. The chart recording mechanism was adjusted to zero so that corrections for tare weight would not have to be made later, and the machine was started. The separation continued for a sufficient distance to ascertain the peel strength, or at least half of the distance of the adhesive line.
The samples were evaluated by comparing their average peel strength after various aging protocols. The best average load line on the recorded curve was manually estimated on the chart drawn by the machine's autographic pen. This value is the actual peel strength, as the machine was calibrated to account for tare weight. The tensile strength tester is accurate to within +/- 5 g.
In this investigation, the color of the samples was measured before and after several stages of accelerated aging in order to determine yellowing, which may indicate instability or degradation.
For each dry mount tissue and aging protocol, four replicates were made. Replicates were cut from the same sheets of dry mount tissue as those used in the peel strength evaluation. Reflection densitometry measurements for blue diffuse density were made in four randomly chosen locations with the status A blue filter of a MacBeth Transmission Reflection Densitometer, model TR-924, located in the Photographic Conservation Laboratory at Old College. The status A blue filter was used because it reads the optical density of yellow, presumed to be the color most likely to change during aging. A polyester template was used to identify the location of the readings so that values obtained before and after aging would be taken from the same location. Each replicate was randomly assigned to an aging protocol.
The samples were aged at 147 F (64 C) +/- 1 C at 86% +/- 2% relative humidity for 0 days, 7 days, 14 days, 21 days, and 28 days in a sealed desiccator in a forced air oven. The samples were placed on a piece of Whatman filter paper and covered with silicone release mylar during aging. A potassium nitrate saturated salt solution was placed in the bottom of the desiccator to insure a constant relative humidity.
The samples were evaluated by comparing mean density change of the yellow values provided by the densitometer for each combination of dry mount tissue and aging protocol.
Using a BMDP 7D computer software package, the data generated was statistically analyzed with Terry Reedy, Statistics Consultant, and Chandra Reedy, Assistant Professor at the University of Delaware/ Winterthur Art Conservation Program. A one way analysis for variance (ANOVA) was used to evaluate the PAT fade and stain tests. Two way ANOVA was used to evaluate the yellowing change by dry mount tissue and aging protocol, as well as to evaluate the peel strength by dry mount tissue and aging protocol. Finally, a one-way ANOVA was used to evaluate the peel strength by dry mount tissue only.
The statistical evaluation proposed above was suggested by Terry and Chandra Reedy, with input from the staff of the Statistics Laboratory of the University of Delaware. Because there is so little literature or previous research upon which to base this investigation, there is no way to predict the number of repeated measures and replicates needed to obtain meaningful data; therefore, it was suggested that as large a number as is logistically possible be used.
Both spectral and manual searches indicated that all four of the adhesives tested are either polyvinyl acetate or polyvinyl acetate-ethylene copolymers. The spectra of Colormount and MT 5 both exhibit additional small, sharp bands at 1600, 1619, and 1562 cm-1, indicating the additional presence of some unsaturated, aromatic functionality, possibly a phthalate or benzoate compound as a plasticizer or as part of the polymer molecule itself. The presence of two kinds of ester compounds is also suggested by the well-resolved doublets at 1753 and 1728 cm-1.
Visual examination of the colloidal silver fade detectors revealed the presence of tiny circular orange/brown spots. After consultation with James Reilly, the author of ANSI IT9.2–1988, it was determined that these should not be construed as evidence of chemical interactions between the dry mount tissue and the colloidal silver. Apparently these spots occur frequently when these colloidal silver detectors are used in the PAT, but they are not a result of contact with the samples.
Some areas of the colloidal silver detectors used with MT 5 showed striated areas which were lighter in color than the surrounding areas. It was determined that these striations were physical deformations resulting from contact with the cockled MT 5. It appears that as the MT 5 was incubated, cockling occurred which pushed aside the colloidal silver in some areas. Cockling was observed in all aged MT 5 samples used in this investigation. However, although the physical deformation of dry mount tissues should be considered, it is not cause for failure of the PAT.
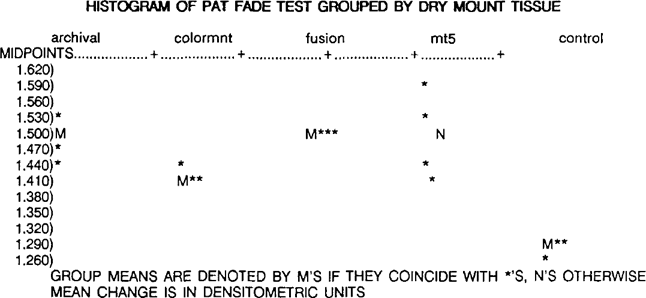
According to ANSI IT9.2–1988, a material will fall the fade portion of the PAT if the mean change in the density readings for each sample is greater than the control fade value, calculated as 1.361 densitometric units. By definition, all of the dry mount tissues tested failed the fade portion of the PAT. The overall ranking, from least to most mean change, is control (1.285), Colormount (1.417), Archivalmount plus (1.491),
MT 5 (1.498), and Fusion 4000 plus (1.498). It should be noted that these samples failed the fade portion of the PAT by a very small margin.

A one way ANOVA for the fade portion of the PAT indicates that all four of the dry mount tissues behaved in essentially the same way, although they are all significantly different from the control (p value < .01). It is interesting to note that there is no variation in the Fusion 4000 plus replicates, and there is large variation in the MT 5 replicates, possible because some of the replicates were obtained from samples of unknown age, or because the colloidal silver was affected by the heat from the tacking iron which was used to remove the dry mount tissue from the detector (see above).
According to ANSI IT9.2, a material will fail the stain portion of the PAT of the mean density change for each sample is greater than the control stain value, calculated as 0.154 densitometric units. By definition, all of the dry mount tissue samples passed the stain portion of the PAT. The overall ranking, from least to most mean change, is MT 5 (0.095), control (0.104), Fusion 4000 plus (0.108), Colormount (0.121), and Archivalmount plus (0.149).
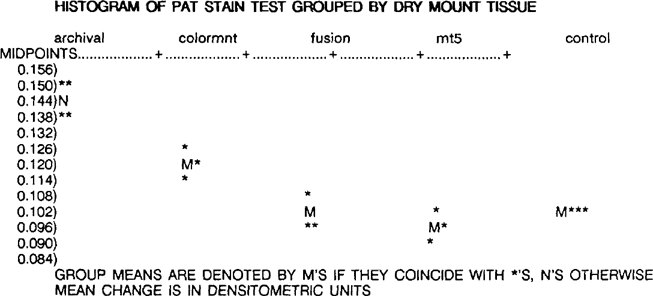
A one way ANOVA for the stain portion of the PAT indicates that the dry mount tissues exhibited significantly different behavior from one another. The internal replicate variation is essentially of the same magnitude for all of the dry mount tissues, and all four of the control replicates are identical.
After the data and detectors were examined by James Reilly, he concluded that, although the samples failed the fade portion of the PAT, there is actually little evidence to suggest that these dry mount tissues would have deleterious reactions with black and white photographic materials. This conclusion was made for the following reasons: 1) the filter paper control is not really suitable for comparison to non-hygroscopic materials or impermeable materials, and mylar might be a better choice, 2) dry mount tissue is not designed to come into contact with the binders and final image materials of photographs, and a more representative choice might be to use an interleaving paper between the dry mount tissue and the detector.
The peel strength readings for the samples which underwent no artificial aging were compared. The dry mount tissues were ranked by overall average peel strength, and, in order from strongest to weakest, are MT 5 (53 g), Archivalmount plus (47 g), Fusion 4000 plus (30 g), and Colormount (13 g).

A one-way ANOVA for peel strength by paper, using only the unaged samples, indicates that the difference in peel strength between the dry mount tissues is significant (p < .03). It is interesting to note that the Colormount and Fusion 4000 plus are the same for all replicates, and that the MT 5 is quite variable between replicates.
Unfortunately, it is impossible to completely compare the data from subsequent aging protocols because of sample failure. Apparently the bond strength increased in the Archivalmount plus and Fusion 4000 plus samples to an extent which resulted in the failure of the sample instead of the adhesive. This may or may not indicate increased peel strength, or it may be a result of thermal aging. The elevated temperature might have caused the adhesive to soften and creep further into the sample, creating a stronger mechanical bond. The Fusion 4000 samples failed consistently at the flexible member, causing the Nomex to tear. The Archivalmount did not fail in a consistent pattern, and failure was exhibited in the flexible member, the rigid member, within the dry mount tissue itself, and in various combinations of the above. Local delamination was also observed in the aged Archivalmount plus samples. It is interesting to note that the samples that did not exhibit failure, Colormount and MT 5, were those made from adhesives containing the unsaturated, aromatic functionality, as well as the ester compounds.
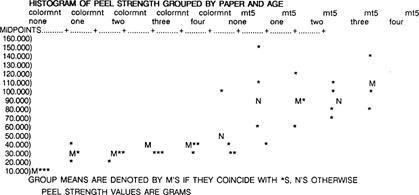
A two-way ANOVA for peel strength by paper and age was used to evaluate the Colormount and MT 5, since they are the only dry mount tissues with complete data. Both Colormount and MT 5 change with age, and MT 5 is much more variable between replicates. The difference between the dry mount tissues is significant (p < .01), and the differences between the aging protocols is significant (p < .02); however, the interaction between the dry mount tissue and age is not significant. Thus, there is a difference between dry mount tissues, and there is a difference between aging protocols, but this difference does not depend on age and vice-versa.
The mean change in densitometry readings was calculated in unaged and aged samples, and the samples were ranked for overall yellowing behavior. From least to most yellowing, they are Fusion 4000 plus, MT 5, Colormount, and Archivalmount plus.
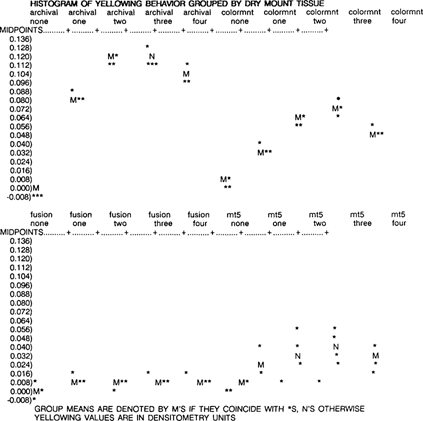
A two-way ANOVA for yellowing change by dry mount tissue and aging protocol was used to evaluate the data. Fusion 4000 plus yellows only slightly after initial aging, then remains stable. MT 5 also increases only a little during aging, but is much more variable between replicates. Colormount yellows progressively as it ages, then yellowing decreases slightly. Archivalmount plus yellows a great deal with age, then decreases slightly. The dry mount tissue, aging protocol and the interaction between dry mount tissue and aging protocol are all statistically significant (p < .01 for all three factors). Thus, there is a difference between dry mount tissues, there is a difference between aging protocols, and the dry mount tissue difference depends on the age and vice-versa.
The adhesives used in the manufacture of these dry mount tissues are very similar in composition. Differences in their aging behavior may be attributed to differences in their paper supports, to additives, or to subtle differences in the polymer molecules themselves.
Although the dry mount tissues failed the strict criteria for passing outlined by ANSI IT9.2–1988, the results of this investigation indicate that there is little evidence to suggest that contact between the dry mount tissue and black and white gelatin photographs would be harmful. A modified PAT, adapted for dry mount tissues and similar materials could include a mylar control and an interleaving layer between samples and detectors, because dry mount tissues are not designed to come into contact with the surfaces of photographs. Perhaps, a more realistic pass/fail criteria could be included in these modifications, because few materials can be expected to perform as well as the filter paper or mylar controls. In any case, the PAT should be repeated for these samples, because of the problems induced by the application of heat and solvents to the detectors after incubation.
There is some suggestion that the peel strength of dry mount tissues increases in these samples. Again, it must be emphasized that these samples are not typical of photographic mounts; furthermore, the sometimes poor correlation between the results of natural and artificial aging must be considered before definitive conclusions about peel strength can be made. Apparently, the behavior of the samples used in this investigation may begin to confirm the empirical observation that the adhesive strength of dry mount tissues does not greatly decrease with age, and that the presence of both an unsaturated, aromatic functionality and ester compounds may have some effect on this behavior.
There are significant differences in the yellowing behavior of these dry mount tissues, and some of the yellowing that was induced would be problematic for photographs on thin paper supports (the kind likely to be dry mounted). For the purposes of empirical observation, a piece of yellowed Archivalmount was placed under half of a cyanotype made with a relatively thin paper support. The discolored dry mount tissue was visible to the eye from through the photograph's primary support. Further investigation is needed to determine if the source of discoloration is the dry mount tissue's adhesive or its paper core.
In addition to the areas of research suggested above, other concerns about the use of dry mount tissue need to be addressed. They include the solubility of dry mount tissue adhesives in new and aged materials, the effects on photographs of the heat and pressure needed to bond dry mount tissues, and the effects on photographs of solvents used to remove dry mount tissues.
I would like to thank the following individuals for their help with this project: Janice H. Carlson, Richard Wolbers, Chandra Reedy, Terry Reedy, Constance McCabe, Ralf Tschirschnitz, and Barbara Lemmen.
(1) Bloede, V. G. Photographic Mosaics 1870, 33–36.
(2) Robinson, H.P.; Abney, W. “The Art and Practice of Silver Printing”; The American Edition: New York, 1881; p 111.
(3) Maddox, B. International Bulletin for Photographic Documentation of the Visual Arts 1987, 14, 36.
(4) Jirat-Wasiutynski, T. The Journal of Audiovisual Media in Medicine 1984, 7, 51–58.
(5) Lyons, R. “Abstracts of Papers”, Fourth Annual Winter Meeting of the Photographic Materials Group, Louisville, Ky., Jan. 1984; American Institute for Conservation: Washington, D.C, 6.
(6) Buchberg, K. “A Comparison of Three Heat-Set Tissues/Dry Mount Resin Formulas”, unpublished paper, New York University; New York.
(7) “American National Standard for Imaging Media- Photographic Processed Films, Plates, and Papers- Filing Enclosures and Storage Containers”, American National Standards Institute 1988, No. ANSI IT9.2–1988.
(8) Schenck, K.; McCabe, Constance. “Issues in Photographic Preservation”, Ninth Meeting of the Photographic Materials Group of the American Institute for Conservation, Cincinnati, Ohio, June 1989; American Institute for Conservation: Washington, D.C., 1989.
(9) McCabe, C., National Archives, personal communication, 1989.
(10) Severson, D., Art Institute of Chicago, personal communication, 1989.
(11) Landrock, A. “Adhesives Technology Handbook”; Noyes Publications: Park Ridge, N. J., 1985.
(12) Allen, K., “Adhesives and Consolidants”, Meeting of the International Institute for Conservation, Paris, Sept. 2–8, 1984, International Institute for Conservation: London, 1984.
(13) Down, J. “Adhesives and Consolidants”, Meeting of the International Institute for Conservation, Paris, Sept. 2–8, 1984, International Institute for Conservation: London, 1984
(14) Bradley, S. “Adhesives and Consolidants”, Meeting of the International Institute for Conservation, Paris, Sept. 2–8, 1984, International Institute for Conservation: London, 1984.
(15) “Standard Test Method for Peel or Stripping Strength of Adhesive Bonds”, American Society for Testing and Materials 1983, No. ASTM D 903–49.