Topics in Photographic Preservation 1991, Volume 4, Article 7 (pp. 96-105)
This paper discusses some aspects of the problem of the evaluation and selection of appropriate paper materials for photographic collections. The topics covered include the chemical and physical properties that affect product selection; the interpretation and description of standard testing methods; and a summary of a number of recommendations for the purchase of suitable materials.
As a background to these subjects, it would be useful to briefly outline some of the reasons why products based on paper fibres make good storage materials. The first point is that paper has a tremendous capacity to buffer against changes in relative humidity. For example, if a photograph is placed in a cardboard box or wrapped in several layers of tissue, it will not respond to changes in RH nearly as quickly as it would if it was more exposed to the atmosphere. Secondly, acidic and oxidative air pollutants do not readily pass through cellulosic material. Instead, they tend to react or complex with the cellulosic substrate and hence the artifact is protected. Third, if properly selected, cellulosic storage materials are extremely stable. Furthermore, our knowledge of their stability is not based on some theoretical conclusions arising from accelerated ageing tests but rather may be drawn from observations made of paper and textile material which has successfully survived many centuries.
An evaluation of paper products for photo collections involves the collection of two types of information:
1. The first involves knowledge of the durability of the material. As a parameter of paper, durability refers to the ability of a particular paper product to remain usable over its expected or desired life-time. Materials used for different purposes will have very different durability and physical requirements. For example, stiffness is necessary in a box but not in an inter-leaving tissue.
2. The second area to consider is the permanence of the fibres. The use of criteria for permanence is a good way of looking at the issue of long-term durability because they address how stable the paper is and to what extent it retains its important chemical and physical properties over time.
Overlapping with the permanence question is the performance of tests geared to the detection of components which can be harmful specifically to photographs.
All of these types of information involve some type of paper testing. The section below describes some of the procedures that can be used to obtain information that is useful for the evaluation of paper. The standard methods cited may be obtained from the addresses given in the APPENDIX.
The most simple testing methods involve basic measurements like grammage (TAPPI T410 om-88; TAPPI TIS 0808–01) or calliper (TAPPI T411 om-84). They give information about the weight of the paper but do not give a great deal of information that is useful in predicting durability. In estimating durability, it is critical that the paper scientist chose tests which measure whatever property is required for the particular use of the product. Conclusions can be highly dependent upon the method of analysis.
The most reliable durability testing involves some type of strength testing. There are many types of strength measurements; a number of the more important examples are as follows:
1. Tear strength (TAPPI T414 om-82 or T496 cm-85; internal tearing resistance) is a measure of the toughness of a paper, the ability to withstand physical shock such as dropping a paper bag that is full of cement. This testing method is not particularly useful as a measure of chemical stability. However, it does give a better indication of brittleness than any of the other tests described in this list.
2. Tensile strength (TAPPI T494 om-88) testing determines the force required to pull a paper sheet apart. A strip of paper is clamped between two jaws, tension is applied and the paper breaks at a point which is descriptive of the strength of the bonding between the paper fibres. In addition to tensile strength (i.e. force per unit width required to break a specimen), the method also measures stretch (percent elongation at break), and tensile energy absorption (energy absorbed per unit area of the specimen before breaking).
3. The data which comes from burst strength (TAPPI T403 om-85 for paper; TAPPI T807 om-87 for paperboard; TAPPI T810 om-85 for corrugated and solid fibreboard) is proportional to the breaking stress (i.e. resistance to rupture) that is defined by tensile strength as well as stretch. Burst, like tensile testing, correlates to inter-fibre bonding and does not necessarily describe end-use performance of the material.
4. Zero span testing (TAPPI T506 wd-83 which has become UM 584) is performed in a manner similar to the tensile test but with the jaws in close proximity so that there is a “zero span” between them. The result is data which describes the inherent strength of the fibre rather than the strength of the inter-fibre bonding. An additional advantage is that it is very sensitive and can be used to measure relatively weak papers. For all of these reasons, zero span tends to be one of the best physical testing methods to monitor chemical deterioration of the fibres, especially when one is attempting to evaluate naturally or artificially aged papers.
Fold endurance (TAPPI T511 om-88) has been used extensively in scientific studies in the field of paper preservation studies. However, it is rarely used by the paper industry. The reason is that data from fold endurance testing is extremely variable and is affected greatly by temperature and humidity. The data obtained may be almost impossible to interpret properly. Furthermore, degraded paper (even that which can still be handled safely if minimal care is taken) tends to be too weak to measure by this method. A recent revision of the ANSI standard for permanent paper (ANSI Z39.48–19XX), has deleted it from the testing scheme.
The advantage of doing more than one type of testing is that one has a better profile of the various strength properties that make up a durable multi-purpose product. Since the vast majority of products produced by the paper industry are items of rather short expected life-time, the emphasis has been on the importance of strength testing new materials and seldom has extended to the question of what happens after years or decades of normal use. Consequently, it is often very difficult to take information from industrial data sheets and make conclusions regarding permanence. The ability to foresee the type and rate of changes in a product is inherent in coming to any conclusion about its permanence.
As with durability, the accurate estimation of permanence will depend upon the choice of testing methods that correctly describe the chemical or physical properties that are important to chemical and physical stability. However, true permanence testing usually involves the analysis of the materials before and after ageing. Without this analysis before and after ageing, strength testing will give only minimal information about permanence.
There are two ways to approach the question of ageing. The most straightforward is to simulate ageing by artificial means. The currently accepted methods of thermal accelerated ageing of paper use temperatures between 70 and 90°C and maintain normal fibre moisture contents by keeping the relative humidity in the 50% range. Degradation during natural ageing is balanced between hydrolytic mechanisms (i.e. involving the participation of water) and oxidative mechanisms (i.e. involving the participation of oxygen). Use of relative humidities greatly higher than 50% during accelerated ageing tends to accentuate hydrolytic break-down at the expense of oxidative deterioration; very low relative humidities do the opposite.
Many valid criticisms can be made of artificial procedures such as moist oven ageing but it still remains our only available way of determining what effect the passage of time may have on a particular material. The most important problems arise when scientists try to translate a specific amount of aging under certain conditions into a precise time period. It is impossible to say accurately what type and amount of ageing is truly equal to 50 or 100 years of time. However, what scientists can say, often with great accuracy, is the relative degree of permanence or stability of a particular set of papers under study. This is done by testing the papers before and after the accelerated ageing, followed by formulating conclusions based on the size of the changes that have resulted.
Although scientists accept the fact that testing before and after accelerated ageing is the best way to determine permanence, it can be difficult to obtain this information about specific products. One way around this problem is to base conclusions regarding the permanence of a specific product on knowledge of what constitutes chemically stable paper fibres. This comes out of data from accelerated ageing experiments of other products as well as evaluation of old naturally aged materials. For example, it is known that 18th century linen paper has fared well over time; therefore, one can conclude that modern 100% linen or cotton will also do well, providing the fibres are not acidic.
There are a number of factors that are important in locating permanent paper products. As the example of the 18th century paper tells conservators, one of the most important considerations is the fibre content of the material. Sources like cotton or linen usually indicate a promising choice. However, these materials are usually expensive and so conservators are sometimes forced to consider wood pulp fibres.
A well processed wood-pulp fibre with all components removed except for cellulose can often be an appropriate choice. The important point is that all of the lignin, hemi-cellulose and resin extractives must be removed. These materials are unstable and make the paper degrade quickly. The extractives and hemi-celluloses are fairly easy to remove and tend not to be present except in very poor quality ground-wood pulps. However, extensive chemical processing must be carried out in order to bring the lignin content down to an acceptable level. No studies have been done which allow scientists to specify precisely what this level of lignin should be. However, it is useful to note that fully processed pulps usually have no more than 0.1 to 0.3% lignin.
Photographs are especially sensitive to lignin in paper. There are three ways in which ligneous fibres may react with photographic materials. The first is through the peroxides which are given off by lignin; these highly reactive peroxides attack the silver image of the photograph. Secondly, partially processed lignin pulps will tend to have high levels of sulphur; break-down of these sulphonated lignins results in compounds which accelerate the deterioration of the image. Third, incompletely processed pulps or mixed-fibre pulps which contain material that was delignified or bleached with chlorine-based oxidizing agents like chlorine dioxide or hypochlorite, may contain significant chlorine residues. (Lignin retains chlorine residues to a much greater extent than cellulose and so incompletely delignified wood pulp is more likely to contain elevated chlorine levels than is a highly purified pulp.) The chemically unstable chlorinated lignins will increase the rate of break-down of the fibres and speed up the deterioration of any photograph that is in contact with the ligneous material.
The lignin content of papers can be estimated by a number of standard methods. In developing their standard for permanent paper, the NISO committee has selected Kappa number (Tappi 236 cm-85) as their method for lignin analysis. This procedure is based on the fact that oxidizing agents like potassium permanganate react with lignin. The disadvantage of this method is that it does not give a direct estimation of lignin but rather gives numbers which correspond to the “bleachability” of specific pulps. It is best used for pulps obtained in yields under 60%.
Another method, the determination of acid-insoluble lignin (Tappi 222 om-88), gives more direct quantitative data and is most frequently used for high yield pulps. This procedure is best suited to softwoods (coniferous) and sulphate pulps which have little acid-soluble lignin (0.2–0.5%). Approximately 3 to 5% of the lignin in hardwoods (deciduous) and sulphite pulps will be soluble (and hence not quantified). At the other end of the scale are the semi-bleached pulps which may contain as much as half of their lignin in an acid-soluble form; TAPPI 222 is obviously unsuitable for these pulps.
Associated with the idea of fibre content is the degree of acidity of the fibres. Acid-catalyzed hydrolysis of cellulose is cited often as the single most important cause of degradation of paper products. Therefore, information on the acidity or pH of a paper can greatly help in estimating its permanence. Lignin is the source of much of the acid in incompletely processed wood pulp papers. Consequently, knowing something about the fibre content of a paper can be an important clue to its acid level.
More precise methods that can quantify acidity are available. One simple method which is frequently used in a conservation laboratory is surface pH taken with a flat-head electrode (TAPPI T529 om-88); this gives the conservator a number representative of the surface of the paper sheet. The degree of porosity and the degree of water-solubility of acidic or alkaline materials present in or on the paper will influence the number obtained. The cold extraction pH method (TAPPI T509 om-88) involves immersing and extracting water-soluble materials from the entire strata of the sheet. Therefore, the data represent an average value for the entire sheet.
From an analytical point of view, pH is not considered to be very numerically accurate. This is because pH is based on a log scale where it is necessary to increase the concentration of acid or alkali by a factor of 10 in order to see a one unit change in the value. It is most sensitive to numerical changes in acid level at pH values around neutrality, that is, pH 7.0. Therefore, a pH value for a particular paper material can only be said to give the conservator an indication of stability. It does not, of course, give any clue as to how the product will perform physically.
The preservation world has responded to this issue of acid and how it can degrade paper with suggestions regarding the removal of acid from products sold as permanent as well as the inclusion of an alkaline buffer reserve. In general, alkaline papers and boards have been avoided by photographic conservators. This is because a large part of most collections contain gelatin or albumen photographs. These protein substrates degrade in the presence of alkali. However, before banning all buffered materials from photographic storage rooms, it might be useful to look more closely at the situation. The alkali used in most cases is calcium carbonate. This chemical does not migrate as acid does but instead, remains fixed to the fibres to which it has been added. Therefore, the real danger is in placing the photograph emulsion in direct contact with the buffered surface. As long as this is avoided, there is no reason why items such buffered boxes cannot be used. However, the best box would probably have a neutral interior with a buffered exterior.
Determination of alkaline reserve involves the accurate estimation of the quantity of buffering salts present in the sheet. This is done by measuring the amount of acid needed to neutralize all of the alkali (ASTM D 4988–89). Once again, it says something about permanence but not necessarily about durability. It is likely that different fibres need different levels of buffering. However, no research results are available concerning what is the optimum buffering level for paper fibres of various types. Until we know more about the subject, the value given by the ANSI standard for permanent paper (ANSI Z39.48–19XX), a minimum of 2% is probably a good estimate to use.
The sizes, fillers and adhesives contained in a paper product can have an immense influence on permanency. The most basic requirement is that all of these additives must be identified. In general, the size used must be chemically stable, acid-free and not contain any alum or rosin (TAPPI T408 om-88). Adhesives and fillers also must be chemically stable and have an appropriate pH. Any dyes or pigments incorporated into the paper should be chemically inert and light-fast.
The type and level of impurities must also be investigated. There are several types of contaminants that should be identified specifically. For example, heavy metals such as copper and iron are known to catalyze the oxidation and deterioration of cellulose fibres. Consequently, permanent paper will have a low concentration of them. Usually this means less than one part per million, although once again, there is no published data to support a definitive conclusion concerning allowable levels.
The paper product must also be free of waxes, plasticizers, and oxidizing chemicals. Nor should it contain any reducible sulphur (TAPPI 406 om-82). As mentioned above, chemically reactive sulphur causes loss of image density on photographs and so this requirement is a particular concern for photo conservators.
Many of the points which have been made in this discussion can be found in the ANSI Standards for permanent paper (ANSI Z39.48–19XX) and for photographic enclosures (ANSI IT9.2 1988). However, we should be aware of the fact that these standards do have some limitations. For example, it is interesting to note that in order to be considered permanent by the ANSI standard for permanent paper the paper must have an alkaline reserve of at least 2%. This means that unbuffered rag papers would not be considered permanent by this standard. This flaw clearly shows that it is important to apply standards to the materials that they were devised for, and not to try to extend their use beyond their intended area.
In order to understand standards such as those devised by ANSI, it is also important to recognize that they are arrived at through a democratic process which involves technical input by their members, compiling by an appointed committee, followed by voting on proposed drafts by all members. Any process like this tends to create a situation where the finished product is not the best nor the worst ideas, but rather what the majority will accept.
The following points may be useful in the selection of appropriate cellulosic materials for photographic collections:
1. Look for products where the manufacturer or distributor will give the consumer some information on such important items as fibre content, pH, and what sizes, adhesives or additives were used. In the case of buffered products, find out what buffering material is used and at what percentage. If the supplier will not or cannot give detailed specifications, it is unlikely that they will also be in a position where they can guarantee the quality of their products.
2. Beware of catch phrases like acid-free, archival, conservation quality, and lignin-free. They can mean many things to many suppliers and their definition may not be yours. A few examples of pitfalls include the following:
♦ “acid-free” could be any pH from 6 up to 11; more descriptive and hence useful terms are “neutral” or “alkaline buffered”
♦ archival or conservation quality could simply mean that the manufacturer would like a conservator to buy their product for preservation purposes; at this point there are only a few standards in this field and they are not being used very widely by the paper industry
♦ lignin-free should mean that the paper does not contain any lignin, whether chemically modified or not; it does not mean that the fibres do not contain ground wood; any lignin (greater than the 0.1 to 0.3% mentioned above) is unacceptable; the fact that a wood fibre paper is labelled as chemically processed, is no guarantee that the product does not contain lignin; many papers contain a mixture of pulps of widely varying qualities.
3. When an order for materials is placed with a distributor or manufacturer, it is wise to make sure that the order specifies exactly what is expected. There may be problems in forcing suppliers to abide by specifications or promises made in their catalogues unless the purchaser also explicitly lists them in the order, along with any pertinent qualifications. For example, when specifying lignin-free material, add the phrase–“free of ground-wood or modified lignins” to avoid any problem with differing definitions of “lignin-free.”
In order to verify important specifications like lignin content and pH, a conservator may wish to carry out some simple testing of the cellulosic products. As mentioned earlier in this article, surface readings (as described in TAPPI T529 om-88) can give useful information but the best data will result from cold extraction values (TAPPI T509 om-88). Archival pens are only adequate for ball-park estimates.
The presence of lignin can be detected with a spot-test solution called phloroglucinol (see APPENDIX). When using phloroglucinol, it is helpful to know that it is possible to get a false-negative result with buffered materials due to the fact that the alkaline buffer in the paper is neutralizing the acid in the test solution. If it is known or suspected that the material being tested is buffered, simply place a little vinegar or dilute acetic acid on the area to be spotted (in order to neutralize the buffer), before the test solution is dropped onto the surface.
False-positive readings are rare. Normally, when one examines a ligneous sample under the microscope, the stain is associated with discrete lignin-rich portions of individual fibres. If the stain is distributed extremely evenly over the entire fibre web (including surfaces between fibres) a size, filler or coating associated with the paper probably is reacting with the phloroglucinol to give a false-positive reading.
Recent improvements in the ANSI photo-activity test make it a powerful tool for the selection of appropriate products for photographic collections. However, the test takes considerable time, expertise and equipment. Therefore, it probably will never be used routinely except in special research and testing facilities which are specially set up to carry the test out on an every-day basis.
A low technology alternative is available which is based on the fact that organic materials are able to react with photographic film; an image is visible after development of the film. This phenomenon was first noted by William Russell who wrote a series of articles on the subject which appeared in the Proceedings of the Royal Society between 18971 and 1908. Vincent Daniels of the British Museum has written three excellent articles2–4 in which he discusses this phenomenon (commonly known under the name of the “Russell effect”). Daniels recommends the procedure as a very useful screening tool for the evaluation of commercial papers or boards that may be used in contact with photographs.
The method is based on the fact that as organic materials degrade, they become oxidized. This oxidation causes the material to give off hydrogen peroxide. Therefore, the rate of evolution of peroxide can give valuable information about the stability and permanence of any organic material under study. The quantity of peroxide that is given off in just a few hours is sufficient to fog photographic film. The extreme sensitivity of the Russell effect allows one to measure easily an ongoing phenomenon of oxidative deterioration. There is no need to resort to artificial means of ageing materials or speeding up of processes. Comparisons between various products can be made based on the extent of their ability to cause this reaction.
In order to obtain a Russell image, the paper material is placed overnight in direct contact with an appropriate sensitized film, in the dark. The film is then developed with a conventional fixer and developer. The density of the image obtained will be proportional to the amount of peroxide given off.
One of the authors (Carolyn Leckie, an ethnographic conservator at CCI) investigated the Russell effect as her Master's project at Queen's University.5 The aim was to see if the procedure could be used easily for routine use in a conservation lab. The following section briefly summarizes a few points which will be of help to a photo conservator in setting up the technique in their laboratory.
The procedure for obtaining Russell images involves three steps: sensitization of the film, exposure of the film, and development of the film. The most important single requirement in obtaining good results is to use the correct film. The author (C.L.) followed the recommendation in Vincent Daniels' first publication2, and obtained excellent success with Kodak type 2566 film. However, Daniels reported in a subsequent publication3 that the formulation of the British film has changed; he now recommends that people in the United Kingdom purchase precision line FP4 film. The Kodak 2566 manufactured in the USA has not been altered and can still be used for obtaining Russell images.
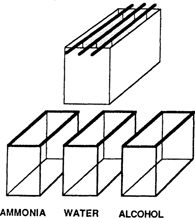
Daniels' papers indicate that a safelight can be used for the sensitization process. However, the author found that the best and easiest results are obtained by doing all operations completely in the dark. The film is sensitized by immersion in 3% v/v ammonium hydroxide plus 0.01% Kodak Photoflo for 5 min. An apparatus such as the one shown in Figure 1 can be used. After immersion in the ammonia, the films are transferred to tap water for 2 minutes and then 50% ethanol for 5 min. All operations are done at room temperature. The ammonia and water are used only once, the alcohol is replaced when it becomes discoloured. The wet film is dried in the dark with careful attention to the exclusion of light. A print drver can be used after 10 min of air-drying as long as room temperature air is used.
Film sensitized on different days can show small differences. Therefore, if it is necessary to compare a large number of paper samples, it is best done on film sensitized in the same batch. In short, the key to good results with this method is rigorous attention to reproducibility of times and conditions of sensitization of the film. Making up large quantities of stock solutions (from which many batches can be processed) can be of great help, as can the use of racks to sensitive several sheets of film simultaneously (Figure 1). The sensitized film should be used up within 2 or 3 days. It should, of course, be stored with complete exclusion of light.

Figure 1: Sequence of solutions used in batch processing.
The exposure of the film also takes place in the dark. The procedure is to simply place the flat samples of cellulosic materials in direct contact with the sensitized film. They can be held in place with glass plates or though the use of apparatus such as X-ray cassettes. Some experimentation may be necessary in order to determine the optimum exposure times. Organic materials typically require between 18 and 48 hours. Care should be taken not to over-expose the film as this can give misleading information (e.g. the image may appear to bleach).
After exposure, the film is developed for one minute in full strength Kodak D19, rinsed in Kodak indicator stop bath, and then fixed in Ektaflo. The film is washed for at least twenty minutes and then rinsed in Photoflo before air-drying. Vincent Daniels' 1984 paper2 includes a photograph of Russell images for eight different mounting boards. The results show a good correlation between the quality of the board and the Russell image obtained.
It is a good idea to include two standard sample papers, along with the “unknowns”. One should be a very good quality rag fibre material, preferably one which has done very well on the ANSI photo-activity test. The second should be a very poor quality ligneous ground wood paper. These two samples will give the upper and lower ranges and provide a basis for comparing the unknown samples. A peroxide gradient which can be used as a reliable standardization for the system has also been devised. The full details are given in the Queen's University project report and will be submitted to Studies in Conservation.
The work on the Russell effect was carried out at the Carnegie Museum of Natural History under the direction of Steve L. Williams, Collections Manager, with the help of IMS Conservation Project Grant IC-70200–87. The authors would also like to acknowledge Cliff McCawley, Chief, Conservation Processes Research, CCI for his helpful comments during the writing of this manuscript.
1. Russell, W.J., “On the Action Exerted by Certain Metals and Other Substances on a Photographic Plate.” Proc. Royal Soc., London, vol. 61, 1897. pp.424–433. A complete list of Russell's articles is available from the references cited in Daniels article (Reference #2 below).
2. Daniels, V., “The Russell Effect - A Review of Its Possible Uses in Conservation and the Scientific Examination of Materials,” Studies in Conservation, vol. 29, 1984, pp. 57–62.
3. Daniels, Vincent D., “Monitoring the Autoxidation of Paper Using Photographic Materials,” in “Historic Textile and Paper Materials: Conservation & Characterization,” in H.L. Needles & S.H. Zeronian, eds., Advances in Chemistry Series #212 (Washington: American Chemical Society, 1984) pp.317–327.
4. Daniels, Vincent D., “The Russell Effect and Its Uses in Non-Destructive Testing,” Phil. Trans. R. Soc. Lond., 1986, pp. 1–9.
5. Leckie, C.G., “The Application and Interpretation of the Russell Effect for Museum Processes,” Project Report for Master's of Art Conservation, Queen's University, Kingston, Ontario, Canada, 1989.
The TAPPI Standards referred to in the text are available from the Technical Association of the Pulp and Paper Association (TAPPI) located at Technology Park, P.O. Box 105113, Atlanta, Georgia 30348–5113; telephone: (404)446–1400.
The ASTM Standards are available from the Annual Book of ASTM Standards, published by the American Society for Testing and Materials (ASTM) 1916 Race Street, Pennsylvania 19103; telephone 215(299–5400).
The ANSI Standards are available from the American National Standards Institute, Inc., 1430 Broadway, New York, NY 10018.
Historically, many of the standard testing methods that were available from TAPPI were also included in the ASTM standards. In many cases, the technical content was similar with only small differences in publication style and format. More recently, ASTM has discontinued publishing their own standard for these topics; instead, the TAPPI standard is included in the published Annual Book of ASTM Standards. In order to avoid unnecessary cross-referencing, the TAPPI numbers (only) are given for citations that appear in both publications.
The recipe for the phloroglucinol staining solution is given below (taken from “Analysis of Paper,” 2nd Edition, by B.L. Browning (Marcei Dekker: New York, 1977) p. 73.
“Phloroglucinol (1 g) is dissolved in a mixture of methanol (50 ml), concentrated hydrochloric acid (50 ml), and water (50 ml). The solution should be protected from light. It slowly yellows with time.”
A more detailed discussion of stains, including the phloroglucinol solution may be found in TAPPI 401 om-88, “Fiber Analysis of Paper and Paperboard.”