Topics in Photographic Preservation 1991, Volume 4, Article 8 (pp. 106-123)
Very popular at the turn of the century, aristotypes are the last POP processes but their industrial coating made them the precursors of modern photographic papers. Intensively used during twenty years, they progressively disappeared for various reasons. It is difficult to get two prints alike, as uncontrollable factors such as light, humidity and regularity of treatment come into play. Moreover, these direct darkening emulsions need a lot of light, and exposure time can be long in winter or when there is no sun. To remede this, “gaslight” papers were commercialized: these silver chloro-bromide emulsions are more sensitive. Exposure is done using artificial light and the image is then developped. An “aristogène” developer was put on the market from 1893 in order to develop aristotypes when the daylight is not sufficient.
The decrease of negatives size put an end to these processes. The contact prints are the same size as the negatives and are easily viewed, being 9 × 12 cm. This is not possible with 35 mm negatives. Since the photographer would have to enlarge the negative, he decided to use development paper.

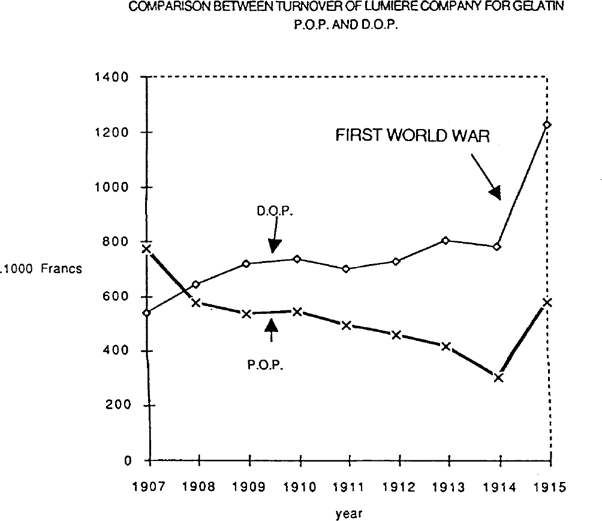
FIGURE 1: COMPARISON BETWEEN TURNOVER OF LUMIERE COMPANY FOR GELATIN P.O.P. AND D.O.P.
The name ‘aristotype’ was used indifferently for both gelatin and collodion base photographic paper.
Chronologically one is inclined to call ‘aristotype’ the collodion based process. It was under this name that E. LIESEGANG put on the market a collodio-chloride paper in 1884. A year later the gelatino-chloride process appeared. These two papers were very similar and were taken for one another. In 1897. the French Legros (16) suggested in his book, to name “aristotype emulsion” the collodion process and “aristotype paper” the gelatine process. Other names have been used.
GELATINE P.O.P.: |
COLLODION P.O.P.: |
ARISTOTYPE |
ARISTOTYPE |
GELATINE-CHLORIDE PAPER |
COLLODIO-CHLORIDE PAPER |
CITRATE PAPER |
CELLOIDINE PAPERSILVER PYROXYCHLORIDE PAPER |
From a technological point of view, the great innovation of aristotypes resides first of all in the use of an emulsion. The sensibilisation of the base is no longer done by successive soakings but by coating with a solution containing suspended photosensitive salts. The paper support is subject to a treatment; baryta coating. This method, patented in 1881, by Hutinet and Lamy (15) and designed to give a glossy surface to the photograph, consists in giving to the paper a coating of barium sulfat in suspension in a gelatin, arabic gum or albumin solution. Gelatin is the most commonly used binder. With the time the formulation evolved and manufacturers introduce wax, casein, milk, starch…etc.
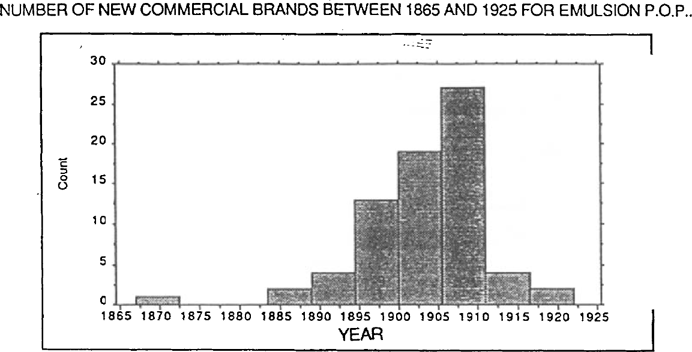
FIGURE 2: NUMBER OF NEW COMMERCIAL BRANDS BETWEEN 1865 AND 1925 FOR EMULSION P.O.P.
The use of a collodio-chloride emulsion to prepare photographic paper was first proposed in March 1865 by Wharton Simpson (28). Noticing that the silver chloride remains in suspension in the collodion he covered a sheet of paper with this preparation and obtained positive prints. He also noticed that the results could be modified according to the quantity of silver salts initially introduced. One of the advantages of this paper is that it can keep its sensitive properties much longer than albumen paper and so be produced on an industrial scale.
An emulsion printing-out paper was put on sale in 1866 in Paris (it was leptographic paper) and in 1867 by J.B. Obernetter of Munich but they met a relatively modest success (13) (27). After 1880, the situation evolved. Silver-glass plates negatives were industrially made and delivered ready for use. Amaters became interested and found aristotypes an easy way to obtain positive prints. In 1884 the Liesegang and then E. Obernetter company successfully launched aristotype papers. There are many advantages; a greater sensitivity (twice that of albumen paper) (2)(11) (13), a consistent quality, ease of treatment (fixing-toning in one bath) a glossy surface, and good resolution. Finally, compared to albumen paper which yellowed in less than ten years, aristotypes seemed permanent (17).
Towards 1893, to answer the aesthetic demands of the time, matt collodion papers appeared. Using a toning bath, the soft tones of the platinotypes that the price of platinum made too costly, can be reproduced.
At the turn of the century, collodion self toning papers are soldd, the emulsion containing gold or platinium salt.
The preparation of the gelatino-chloride papers was perfected by Abney in 1882. Their industrial production started in Germany in 1885 with E. Obernetter (son of J.B. Obernetter) followed by Liesegang (8). It closely followed the production of collodio-chloride papers and avoided two phenomena; the curling of the paper in the treatment baths and the detaching or scratching of the collodion binder during handling.
The first aristotypes were hand coated, the emulsion being poured on a sheet of paper already fixed in a frame or fixed by three angles on a wood plate. However, very soon, the coating was automated using a cylinder machine (27).
Some other processes can be affiliated to aristotypes
Silver chromate paper was patented 1898 by Ferdinant Hrdlicka in Vienna. This new collodion emulsion contains silver chromate and was used for weak negatives. It was sold under the name of Rembrandt paper (8) (27).
Starch aristotypes are put on the market by the Marion Company at the end of the 19th century. The gelatin is replaced by a mixture of starch and dextrine. They are baryta coated, smooth or grainy and matt (9).
A paper, published in 1904 by the French Society of Photography issue, recommended arabic gum as a binder to replace starch (23). There is no evidence that was produced industrially.
Casoïdin paper is a casein based emulsion invented by Otto Buss in Switzerland and produced by O. Wilde in Goerlitz (Germany) then by Gevaert in Antwerp (Belgium) in 1905 (27).
Protalbin paper was invented by Jolles and Lilienfeld in 1897 in Vienna. It was manufactured after 1900 by Wiener Chemische Werke and Protalbin Werke (Dresden). The binder is made using an alcohol solution of protides extracted from cereal grains and mixed with albumin, collodion or gelatin (8) (27).
At the end of the 19th century several companies (Lamy, Marion, Lumière) produced aristotypes whose emulsion, gelatin based, is coated on paper which has not undergone a baryta coating.
All of these sensitive surfaces were used in a similar fashion, the image was formed photolytically. Several theories were used to explain the formation of the image.
In 1849, E. Becquerel came up with an explanation that met with widespread approval in scientific circles and was taken up by different authors during more than fifty years. According to this theory a compound called silver sub chloride (Ag2 Cl) is formed under light:

During fixing silver sub chloride is decomposed into metallic silver and silver chloride which is dissolved in the fixing solution. However no one was able to isolate this salt. In 1887 when Güntz (12) showed the existence of a silver sub fluoride salt (Ag2 F), the sub-chloride theory found a certain recognition and Carey-Lea (24) became an ardent defendor of the theory.
Other scientists such as Davanne and Girard, then Lumière and Lüppo-Cramer after, rejected the theory thinking that a complex silver colloidal-silver chloride(Ag-AgCl) is formed. New methods of analysis such as X rays diffraction put an end in about 1930, to the sub-chloride theory showing that the action of the light provokes the formation of colloidal silver (24).
When a gelatino-bromide paper and an aristotype are exposed to daylight the aristotype darkens quickly whilst the bromide paper turns a light grey. This difference is due to the presence of silver nitrate in excess in the POP. This react with chlorine given off by the decomposition of the silver chloride and photosensitives silver chloride is formed. In the bromide paper, the absence of compound able to capture the bromine, limits the darkening because the reversed reaction happens: the halogen reacts with the silver.
The greatest part of the silver in an aristotype is found in the form of soluble salts. Meidinger (21) and Weigert (25) estimated at only 30% the proportion of silver chloride and 70% the mixture of silver nitrate and silver citrate (or tartrate). These soluble salts (silver nitrate and silver citrate) play an important rôle in the image formation and in the silver deposition. The silver citrate acts as a spectral sensibilisator. This whas show by the work published in 1882 by Abney (2). He recorded the curves of the spectral sensivity which indicated that the silver citrate (or tartrate) permitted to extand the sensivity of aristotype papers to visible light. The silver chloride, alone, is sensitive to ultra-violet rays.
After exposure, the samples are washed, toned with gold or platinum, then fixed. After 1914, the price of these metals increased dramatically and photographers tried to replace them with less expensive compounds producing satisfactory hues.
Lead salts are able to produce good violet tones but the image is less permanent.
The Lumière brothers showed that lead cannot be used for a toning bath except if mixed with gold salts (19). Other metals like uranium, copper, mercury, osmium and iron can be used to tone the aristotypes and give various tones (5). However only toning using selenium gives good results at an affordable price and garanteeing long term preservation (20).
The fixing bath removed the residual silver salts. When the sample is immerrsed, a fading and change of tone can be observed. These two phenomena, which do not occur with development paper, have several physical and chemical origins. The color shift is due to the transformation of the silver colloidal-chloride complex into silver gelatin (or collodion) whose maximum absorption is situated at a short wavelength. The dissolving of the silver chloride leaves space in the binder where the silver particles can rearrange themselves and coagulate, which modifies their optical qualities (10) (18) (21). The pH of the fixing bath is of great importance. When the paper is fixed the citric acid, already present in the emulsion, acidifies the solution. In an acid environment the sodium thiosulfate is decomposed to sulfide which reacts with the silver. The image is submitted to a sulfur toning, which modifies the hue. Moreover, the acidified thiosulfate is a solvent of silver, and these effects are most noticeable in the areas of low density.
The use of a fixing-toning bath allows the realisation of both operations simultaneously. This bath simplifies the operation but some authors suspected it to be the cause of deteriorations (3) (4) (7). The work of Lumière and Seyewetz showed that the main factor causing the degradation of the images is the presence of residual thiosulfate(19).
As soon as they were produced aristotypes were announced as permanent processes. They are, it is true, much more stable than the albumen papers but, according to several authors, such as Deck (6) (7) or Hanneke (14) their stability is due mainly to the way in which the images are treated, handled and stored.
Collodion aristotypes are mechanically fragile; they scratch easily and the image layer can sometimes leave the support. Collodion is not very sensitive to water and so cannot follow the dimensional variations of the paper when it is placed in a humide atmosphere. The photo tends to curl and the image cracks. It also occurs that the cracks are linked to a poor preparation of the barium sulfate layer (26).
Deck (7) noted that, in unfavourable conditions POP alter more quickly than DOP. The small size of the silver particles that form the aristotypes are the reason for this. For the same quantity of silver, the specific surface in contact with the exterior is greater in a POP than in DOP.
The reactions take place in the interface of the metallic particles. In a POP the silver is attacked more quickly.
In order to better understand the structure and mechanism of image deterioration we decided to follow the microstructure of the silver deposit during accelerated ageing.
For this, we prepared samples from old prints and from aristotype paper currently manufactured in France by the Guilleminot Co2. Cross sections were observed under an transmission electronic microscope and the first observations revealed that the size and the shape of the particles varied greatly from one process to another.
This could have resulted from differences in the technical preparation: the compounds used were not always the same. The gelatin paper occasionally contained citric acid which promotes the precipitation of fine crystals of silver chloride thus the size of the halide crystals has an influence on silver grain size. The effect of using for collodion papers an alcoholic solution and, in others case, an aqueous solution, could also be a source of difference …
The sampling has been done on different density areas. In a low density area, the number of grains (and their size) was smaller than in zones of high density. Given the disparity of the particle size, only a statistical study could indicate the differences in structure. To accomplish this we have counted a minimum of one-thousand particles. To render our measurements more precise and less tedious we have numerised the cross section pictures, which were then analysed morphologically.
The figure 3 give, in the form of a histogram, the frequency of distribution of the particles as a function of their size.
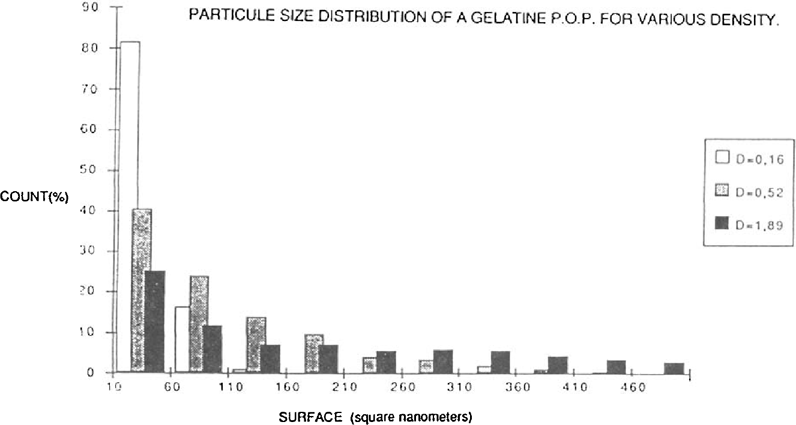
FIGURE 3: SURFACE (square nanometers)
Those with a size between 10 to 60 square nanometers were always in a majority.
In a density area of 0.16 they represent more than 80% of the population. For densities of 0.52 to 1.89 this percentage declines respectively to around 40 to 25%.
The range of size grain increased with the density. Hence, on a density area of 0.52 the size of the particles rarely surpassed 300 square nanometers, in a density area of 1.89 the particles could surpas 1000 square nanometers.
The number of particles by category is not the sole point of interest. In fact, if one counted a great number of small grains, the surface of the image they constitute would be inferior to a category of particles with a larger surface area but lower population. This, which we call “covering rate”, has been applied to each particle category. To obtain this rate, we have totaled the surface of the particles of each category and divided this value by the total surface of all image particles. These results are shown on figure 4. Abscissa indicates the surface averages (average surface of the particles of each category) and the ordinates the covering rate.
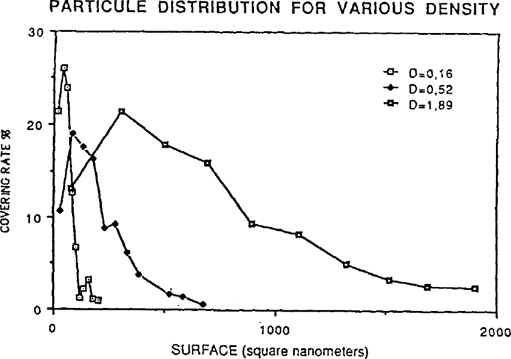
FIGURE 4: PARTICULE DISTRIBUTION FOR VARIOUS DENSITY
The distribution is different from those of figures 3. The silver grains playing the most important rôle in the image constitution are:
A sheet of Guilleminot paper was exposed to the light and then divided in two. One part was gold toned and then fixed and the other simply fixed. The figure 5 show that the gold toned paper contains more small particles than the other (about 20% more).
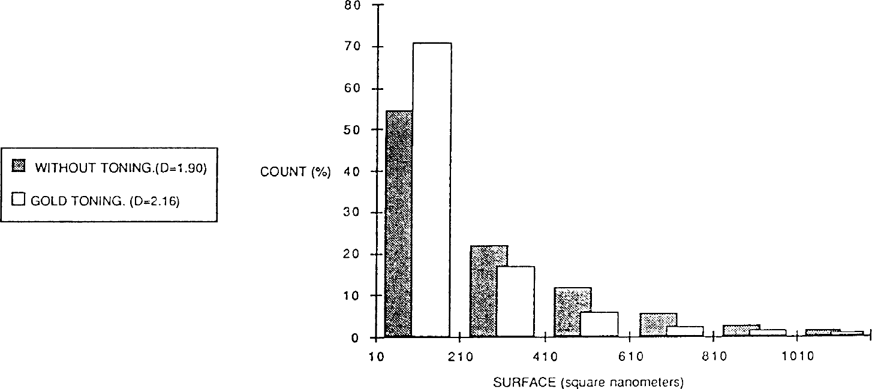
FIGURE 5
These particles play a more important rôle in the constitution of toned photography than in non-toned. Gold toning seems to produce grains of a more irregular form than those particles found on paper non-toned. These visual observations were confirmed with a shape factor measurement.
As the majority of alterations has been attributed to the presence of residual thiosulfate, the aristotypes (gold toned or not) were washed briefly after fixing, in such as a way as to leave a certain quantity of fixing salts in the print. The samples were then hung in a test chamber regulated at 60°C and 70% relative humidity during 31 days. Analysis was carried out according to the same procedure described previously. During the ageing the small grains disappear giving way to medium size particles. The small particles have a reduced importance in the constitution of the image (figure 6).
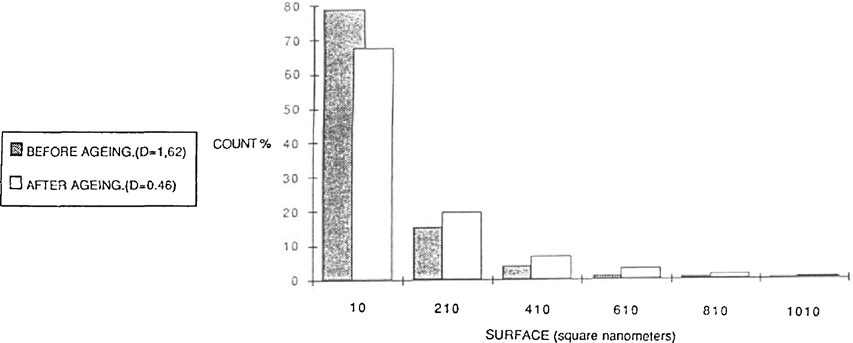
FIGURE 6
The particles have a tendancy to assume a more rounded shape after ageing. Moist-heat ageing was applied to gelatine POP without toning. The results were similar to preceding samples but much more marked. One observes a schift and spreading out of the distribution of the covering rate: thus the preponderant particles are not those of 500 square nanometers but of 700 square nanometers. Once again the smaller particles have disappeared giving way to particles of medium size, or even to larger particles (figure 7).
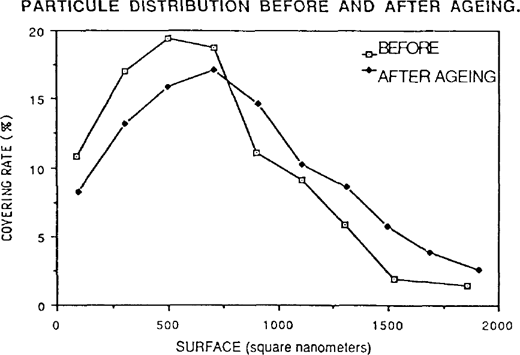
FIGURE 7: PARTICULE DISTRIBUTION BEFORE AND AFTER AGEING.
The shape factor measurement indicates the precise way in which the silver grains have rounded. These calculations confirm visual observation.
The spatial distribution of grains (number of grains per surface unit) was not taken into consideration. In fact, dependent on the thickness of the section (sample) and the inclusion, from the same reference sample we obtained varying results. With a study of a pre-determined number of particles we would be able to follow the changes. Many hypotheses can be forwarded to explain the evolution of the microstructure of the grains after accelerated ageing.
Gold introduced by toning is not solely a deposit of gold on the surface of silver particles: the insertion or substitution of gold into the silver structure could be an origin of these distortions.
The action of auric chloride in an acid environment brings about the replacement of three silver atoms by one gold atom, hence a deformation and reduction in the size of the particles. This explanation is not totally satisfactory. If the size of the particles decreased in such a marked way during toning the results would be a loos of density, however we observed a density increase in relation to the non-toned paper.
To better understand the effects of toning, one should take into consideration all the treatment. With POP, contrary to DOP, the toning takes place before fixing. During this last phase of the operation the silver deposit undergoes profound physical modifications. The silver chloride which bears photolytic silver dissolves leaving enormous cavaties into which the small silver particles settle and fuse. We could have a more extensive idea of this transformation by comparing the amount of silver present on the photosensitive surface (several grams of silver nitrate per square meter) and that remaining after complete exposure and fixing (several tenths of grams of silver per square meter). Observation with an electron microscope of an emulsion reveals a different morphology before and after fixing.
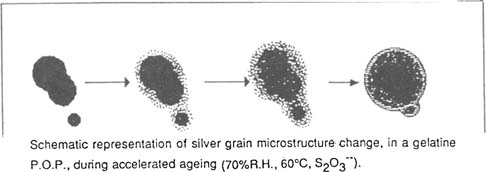
This structure is not an exact reflection of reality because the beam of electrons interacts with the photosensitive salts: we note meanwhile a large number of small grains who were not present after fixing. Gold toning would therefore have consequences with fixing: on one hand effecting the recombing of particles and on the other protecting the silver grains against the corrosion it will under go during fixing. The alterations the photographs undergo during ageing (in the presence of excess thiosulfate) show the extreme mobility of the silver deposit. During ageing the silver grains become round either due to the phenomenum of diffusion, formation of silver sulfide and their coalescence with near by particles or by a transfer of atoms from one particle to another: the ions detach themselves from the smaller particles, migrating to neighboring particles while compact themselves to a more consistent size.
Successive coverings result in spherical deposits. On the photo 2 we notes on the outside of certain particles the presence of a nucleus which bears witness to the anterior existence of a particle. The residual thiosulfate plays without doubt a rôle in the mechanism of oxydo-reduction, the formation of sulfuric acid as the result of it decomposition from moist heat, accelerating the attack on the grain. Meanwhile, excepting these chemical reactions, there exist thermodynamic rules which would explain these transformations.
These results were obtained from high density areas. A future study would enlarge the scope of research by studying low and medium density areas and compare the result with natural ageing.
The microstructure of printing-out papers changes considerably from one photograph to another. This is aligned with numerous factors such as the nature of the binder, the preparation, the treatment, the image density and degradations. The distribution frequency of the silver grains by size and shape allows for a putting into focus the morphological differences amongst silver grains. Gold toning, comparatively to non-toned paper, produces irregular particles as well as a higher number of small particles. Accelerated ageing of paper containing residual thiosulfate in excess induces profound transformation. First of all, one observes the disappearance of the small silver grains giving way to grains of medium size who assume a more perfect circular shape. Gold toning slows down this transformation but does not completely avoid it (22).
This research has been conceived thanks to the suppor given by the Ministère de la Culture: Direction du Patrimoine, Mission du Patrimoine Photographique.
The author would like to thank Françoise Flieder, Chantal Garnier, Marc Germain, Julien de Trébons, Anne-Marie Slézec, Alain Foubert, Dr Klaus B. Hendriks as well as the electronic microscope service of the CNRS, for their wonderful advice and help.

T.E.M. IMAGES : Enlargement :247 500X.
PHOTO I |
PHOTO 2 |
POP GUILLEMINOT |
POP GUILLEMINOT |
REFERENCE |
AFTER AGEING (70% H.R., 6O°C, S203–, 37 days) |
(1) Abney (W.).- Relative spectrum sensitiveness of printing processes and a new form of silver printing. In: The Photographic News, 26 mai 1882, p. 300–301.
(2) Anon;- A new collodio-chloride emulsion. In: British Journal of Photography, 17 avril 1903, p. 302–303.
(3) Baker (T.).- Photographic emulsion technique. Boston: American photographic publishing Co., 1941.
(4) Brigham Foster (W.).- Collodion chloride paper. In: British Journal of Photography, 10 novembre 1905, p. 885–886.
(5) Carteron (J.).- Les virages en toutes couleurs pour tous les papiers. Paris: E. Mazo, 1897.
(6) Deck (C.).- The permanence of photographic prints as tested by tropical climates. In: British Journal of Photography, 13 avril 1923, p. 222–223.
(7) Deck (N.C.).- Stability of photographic prints. In: Bulletin de la Société Française de Photographie, 12, 1925, p. 384–385.
(8) Eder (J.M.).- History of photography. New York: Columbia University Press, 1945.
(9) Ewing (G.).- A Handbook of photography, 2e éd., Calcutta: W. Thacker & C° Editeur, 1909.
(10) Formstecher (F.).- Colour-changes in fixing a print out image. In: Photographische Industrie, 1921, p. 590–591.
(11) Geldmacher (F.W.).- Collodio chloride printing by an improved method. In: British Journal of Photography, 5 février 1886, p. 83–84.
(12) Guntz In: Comptes-rendus de l'Académie des Sciences, 112, 1891, p. 1212; 113, 1891, p. 72.
(13) Hanneke (P.).- Das Celloïdin Papier. Berlin: Verlag von Gustav Schmidt, 1897.
(14) Hanneke (P.).- Stability of photographic prints. In: Photographische Chronik, 45, 1938, p. 71–72.
(15) Hutinet et Lamy.- Procédé de préparation d'un papier photo gélatino bromuré, chloruré, ioduré en vue d'obtenir des épreuves brillantes. Brevet français n° 142141 du 5 avril 1881.
(16) Legros (V.).- L'Aristotypie. Paris: Société d'édition scientifique, 1897.
(17) Liesegang (P.E.).- L'Aristotypie ou le tirage au collodio-chlorure d'argent. In: Le moniteur de la photographie, 1885, p. 124–125.
(18) Lumiere (A. et L.), Seyewetz (L.).- Sur les réactions qui se produisent dans les solutions utilisées pour le virage et le fixage combinés des épreuves sur papier au chlorocitrate d'argent et sur la théorie de cette opération. In: Bulletin de la Société Française de Photographie, 1902, p. 391–402.
(19) Lumiere (A.) et Seyewetz (L.).- Sur l'altération des images imprimées sur papier au chlorocitrate d'argent et fixées en une seule opération. In: Bulletin de la Société Française de Photographie, 1908, p. 481–483.
(20) Lumiere (A.) et Seyewetz (L.).- Sur l'emploi du sélénium comme succédanné dans les virages des papiers à noircissement direct. In: Bulletin de la Société Française de Photographie, 1924, p. 79–80.
(21) Meidinger (W.).- Die Theoretischen Grundlagen der photographischen Prozesse. Wien: Verlag von Julius Springer, 1932.
(22) Reilly (J.M.) et al.- Image structure and deterioration in albumen print. In: Photographic Science and Engineering, 23, 4, juillet-août 1984, p. 166–171.
(23) Reiss.- Préparation d'un papier à la gomme arabique et au nitrate d'argent. In: Bulletin de la Société Française de Photographie, 1904, p. 521–526.
(24) Vassy (A.).- Fondements théoriques de la photographie. Paris: Edition de la Revue d'Optique, 1953.
(25) Weigert (F.).- Photochemistry of silver compounds. In: Ber. Preuss. Akad. Wiss., Berlin, 39, 1921, p. 641–650.
(26) Wentzel (F.).- Cracking of collodion films. In: Photographische Industrie, 1925, p. 87.
(27) Wentzel (F.).- Memoirs of a photochemist. Philadelphie, PA: American Museum of Photographie 1960.
(28) Wharton (S.).- Sur une nouvelle méthode de tirage et sur la préparation et l'emploi du collodio-chlorure d'argent. In: Bulletin de la Société Française de Photographie, 1865, p. 151–157.
1 A more extended paper is published in french In: Les documents graphiques et photographiques. Analyse et conservation. Travaux du Centre de Recherches sur la Conservation des documents graphiques, 1988, 1989. La Documentation Française, Archives Nationales, Paris 1991.
2 GUILLEMINOT, 22 rue de Châteaudun, 75009 Paris, France.