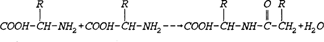
Topics in Photographic Preservation 1991, Volume 4, Article 9 (pp. 124-135)
The use of albumen as a traditional artist's material is long and well established. Cennino Cennini in his Il Libro del' Arte, mentions the use of egg white or “glair” as a binder for pigments and as a varnish. As early photographers sought a suitable medium for the fabrication of prints and negatives, the use of egg white became inevitable. The outstanding result of this initial experimentation is the albumen print.
In general, the term albumen refers to hen's egg white. In the egg, albumen is a relatively pure mixture of numerous proteins dispersed in water.
COMPOSITION OF ALBUMEN |
||||
Proteins |
Lipids |
Carbohydrates |
Ash |
Water |
9.7–10.6% |
0.03% |
0.4–0.9% |
0.5–0.6% |
87.9–89.4% |
As can be seen above, the protein component accounts for the bulk of the solids in albumen. As the water component dries off during albumen paper manufacture, the resulting surface coating is almost entirely protein. After aging, this layer is notably susceptible to a characteristic discoloration and cracking. As a first step towards understanding the properties and degradation behavior of albumen photographs, an overview of the involved proteins and their chemistry may prove helpful.
The major proteins found in egg white (>1% of the total protein content) are listed below.
MAJOR PROTEINS IN ALBUMEN |
% OF TOTAL PROTEINS |
Ovalbumin |
54% |
Conalbumin |
13% |
Ovomucoid |
11% |
Lysozyme (G1) |
3.5% |
Globulins (G2, G3) |
8.0% (?) |
Ovomucin |
1.5% |
Other protein components include, flavoprotein (.8%), ovoglycoprotein (.5%), ovomacroglobulin (.5%), ovoinhibitor (.1%) and avidin(.05%).
Proteins are classified by biological functions and properties. Ovalbumin1 and conalbumin are included under the broad category albumins. It is worth noting that the word albumen (“-men”) refers to the protein mixture derived from egg white while albumin (“-min”) refers only to a specific class of proteins. As a group, albumins are generally soluble in water and dilute saline solutions. They are coagulated by heat and are found in the interstitial fluids of animals. The biological function of conalbumin is to isolate and sequester metallic contaminants in the egg white.
Ovomucoid and ovomucin are classified as mucoproteins. The mucoproteins are usually stable in heat and are easily soluble in water and mild saline solutions. Generally, they can be coagulated by low pH and ethanol. In nature, they occur in skin, bone, blood and eggs. In the egg, ovomucin is responsible for thickening the white.
Globulins is another general classification for proteins. These proteins resemble albumins in that they are coagulated by heat and are soluble in mild saline solutions. As a class they are generally insoluble in water. Like albumins, these proteins occur in the interstitial fluids of animals. Lysozyme, globulin G2 and G3 fall into this category. Lysozyme, which is also found in human saliva, is an enzyme that destroys bacteria.
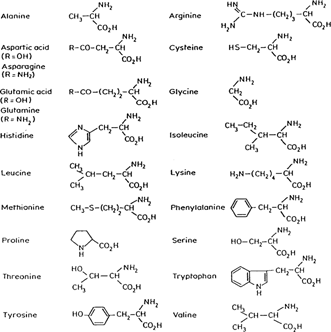
Proteins are made by joining amino acids by what is called a peptide bond. The peptide bond is formed in a reaction between the amino group (-NH2) of one amino acid and the carboxyl group (-COOH) of another amino acid. The resulting linkage yields water. The R-groups in the equation stand for the functional portion of the amino acid which are known as side chains. Proteins (which can include hundreds of peptide bonds) are often referred to as amine acid polymers. Amino acids vary only in the orientation and composition of the R-groups. In nature there exists twenty known amino acids which are diagrammed and listed below (Hardy 1985).
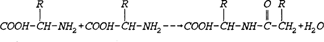
REPRINTED BY PERMISSION
©Chapman & Hall Ltd.
The functional groups of the amino acids are classified by characteristic properties. Measured at, or near, neutral pH, there are polar (hydrophilic) and non-polar (hydrophobic) functional groups. There are also positively charged (basic) and negatively charged (acidic) groups.
The elemental content, amino acid content and amino acid sequence of a protein is known as its primary structure. All proteins contain carbon, oxygen, nitrogen and hydrogen. Proteins usually contain sulfur and, more infrequently, phosphorous. The elemental composition and an amino acid analysis of the major proteins contained in egg white appear on the next page. The first table includes protein molecular weights. The following amino acid tabulations also indicate which proteins are non-polar (np), polar (p), basic (+), or acidic (-). The amino acids which contain sulfur are also indicated [S].
ELEMENTAL COMPOSITION THE MAJOR ALBUMEN PROTEINS as measured by % of dry weight (Young 1963) |
|||||||
Protein |
C |
O |
H |
N |
S |
P |
Mol. wht. |
Ovalbumin |
52.8 |
22.82 |
7.1 |
15.5 |
1.66 |
0.12 |
45,000 |
Conalbumin |
52.5 |
22.07 |
7.0 |
16.6 |
1.83 |
0.00 |
80,000 |
Ovomucoid |
49.0 |
28.80 |
6.9 |
13.1 |
2.20 |
0.00 |
28,000 |
Lysozyme |
48.7 |
23.77 |
6.4 |
18.6 |
2.53 |
0.00 |
14,600 |
AMINO ACID CONTENT OF THE MAJOR ALBUMEN PROTEINS as measured by grams of amino acid per 100 grams of protein (Tristam 1953) |
||||
|
Ovalbumin |
Conalbumin |
Ovomucoid |
Lysozyme |
Alanine (np) |
6.72 |
4.4 |
2.3 |
5.4 |
Valine (np) |
7.05 |
8.2 |
6.0 |
4.8 |
Leucine (np) |
9.2 |
8.8 |
5.1 |
6.9 |
Isoleucine (np) |
7.0 |
5.0 |
1.43 |
5.2 |
Proline (np) |
3.6 |
4.9 |
2.72 |
1.4 |
Phenylalanine (np) |
7.66 |
5.7 |
2.91 |
3.12 |
Tryptophan (np) |
1.20 |
3.0 |
0.3 |
10.6 |
Methionine [S] (np) |
5.2 |
2.03 |
0.95 |
2.06 |
Tyrosine (p) |
3.68 |
4.6 |
3.18 |
3.6 |
Glycine (p) |
3.05 |
5.7 |
3.8 |
5.7 |
Serine (p) |
8.15 |
6.3 |
4.2 |
6.7 |
Threonine (p) |
4.03 |
5.9 |
5.5 |
5.5 |
Cysteine [S] (p) |
1.35 |
- |
- |
0.00 |
Cystine/2* [s] |
0.51 |
3.8 |
6.7 |
6.8 |
Amide N** |
1.02 |
1.04 |
1.0 |
1.71 |
Asparagine** (p) |
- |
- |
- |
- |
Glutamine** (p) |
- |
- |
- |
- |
Arginine (+) |
5.72 |
7.6 |
3.7 |
12.7 |
Histidine (+) |
2.35 |
2.57 |
2.15 |
1.04 |
Lysine (+) |
6.30 |
10.0 |
6.0 |
5.7 |
Aspartic acid (-) |
9.30 |
13.3 |
13.0 |
18.2 |
Glutamic acid (-) |
16.50 |
11.9 |
6.5 |
4.32 |
* Cystine/2 is a measure of two cysteines linked by a disulfide bond and is not itself an amino acid.
** Because of the analytical detection technique used for this particular assay, asparagine and glutamine are destroyed and can not be distinguished or tabulated. Therefore, the totals listed for amide N are combined totals for asparagine and glutamine.
The three dimensional shape of a protein is known as its conformation. The major albumen proteins share a “globular” conformation2. Globular proteins are composed of a rounded, compact mass of an intertwined protein chain. The precise shape of the globular form is due to the interrelations of the component amino acids as they seek the lowest free energy state for the protein. Once the lowest free energy state for a particular protein has been achieved, it is said to be in its native or functional state. Only when in its native, or low energy, state does a protein manifest all of its characteristic properties and biological functions. A conformation diagram for lysozyme is shown below (Dickerson and Geis 1969).
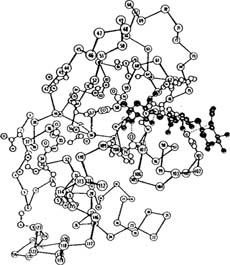
REPRINTED BY PERMISSION
©Irving Geis
There are several considerations which ultimately shape the protein. For instance, hydrophobic amino acids are generally found on the inside of the protein mass and hydrophilic amino acids are found on the outside. The interrelation of adjoining amino acids makes possible only a limited range of bond angles. Also, hydrogen bonding between the amino acids of the same, or between adjacent proteins, will affect the ultimate shape of the molecule. The polar amino acids (cysteine, serine, threonine, asparagine, glutamine and tyrosine) are those that tend to form hydrogen bonds. Another factor influencing the ultimate conformation of a protein is the presence of disulfide bonds, either within a single protein or between two individual proteins. Often referred to as a “disulfide bridge”, this type of bonding occurs between two cysteine amino acids.
The process of altering the native/low free energy conformation of a protein is called denaturation. Denatured proteins do not retain their original properties or biological functions. Denaturation implies only that the specific conformation of a protein has been altered. Peptide bonds generally remain intact and the protein usually retains its original primary structure. Therefore, in principle (though rarely in nature) a denatured protein can return to its native state and resume its specific biological activity.
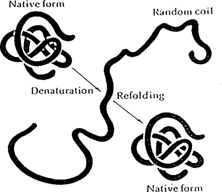
Denaturation/renaturation of a globular protein (Lehninger 1970) REPRINTED BY PERMISSION
©Worth publishing
Proteins have specific parameters for denaturation. These parameters are usually ranges of pH, relative humidity and temperature. Proteins are also commonly denatured by organic solvents and by physically applied stresses. Denaturation can also occur when a protein is exposed to high pressure, radiation and even sound waves.
The proteins found in egg albumen have slightly differing denaturation parameters. Ovalbumin resists thermal denaturation but it is denatured by applying a mechanical force or by exposure to alcohol. Conalbumin is very heat sensitive but is not especially susceptible to mechanical manipulation. The parameters for ovomucoid are such that it resists denaturation by heat in low pH environments but will be heat denatured in more alkaline conditions. Likewise, lysozyme is more readily denatured in alkaline environments than in acidic environments. Though these proteins share slightly differing characteristics, all are susceptible to denaturation when they are dried out of solution.
The preparation of albumen for use as a photographic binder is a denaturation process. In the nineteenth century, photographers and albumen paper manufacturers typically followed modified versions of the same basic procedure when preparing the albumen for use. Starting from 1850, when Louis Désiré Blanquart-Evrard introduced his albumen printing method, it became standard practice to whip the egg white into a froth and let it settle over a time period ranging from overnight to several days. Once settled (and aged, if applicable), the albumen would be strained, usually through muslin.
Based on the denaturation parameters of the albumen proteins, it is evident that all of the main albumen proteins are denatured during processing. In general, allowing the water content of egg white to evaporate during drying is enough to denature the proteins. More specifically, the primary protein in egg white, ovalbumin, is sensitive to the shear forces applied during frothing and becomes denatured due to the stresses inherent in film formation. If alcohol baths or applications of steam were used during the manufacture of albumen paper additional denaturation would occur.
Furthermore, glossier papers, coated with a double layer of albumen, were generally left for months in drying lofts between coats. Temperatures in such lofts could reach up to 50 degrees centigrade (Reilly 1978). Once coated, the paper was often run through a calendaring press to provide additional gloss. The use of heat, and pressure would contribute to substantially alter the native states of the original proteins and, thereby, cause them to become denatured.
Additions of acetic acid to the liquid albumen prior to paper coating and subsequent alkaline gold toning by individual photographers would also serve to further denature the albumen and thereby alter its original properties. The straining of the liquid albumen itself is of significance since it would remove from the final coating those proteins which had precipitated from solution during processing. For example, frothing and straining has been found to lower the overall ovomucin content of egg white (MacDonnell 1954). The extent to which the albumen proteins are altered during manufacture, combined with the aging of the albumen liquid, make it highly unlikely that any of the egg white proteins would become completely renatured over time.
Fermentation is another process which can alter the properties of an organic material. Unlike denaturation, however, fermentation causes irreversible changes within the material. Fermentation takes place when an organic substance is exposed to microorganisms. Yeasts, bacteria and molds are examples of microorganisms that cause fermentation. The action of enzymes is often considered part of the fermentation process. Enzymes are usually present in the organic substrate itself or are secreted by the active microorganisms. During the fermentation process, (1) peptide bonds can be broken, (2) sugars can be digested and altered, (3) and amino acids can be broken down. Yeast for example, which is commonly known for its effect on sugars, can derive some of its nutritional requirement from the amino acid lysine (Nickerson and Rosivalli 1976).
In the processing of albumen for photographic use, fermentation played a role in shaping the final product. After frothing, even if the albumen was left to settle just overnight, the constituent proteins would be altered. Frothing itself contributes to this process by exposing more of the albumen to air. Microbes, like enzymes and proteins, have very specific pH and temperature parameters under which they are active. Therefore, if the albumen was left to ferment over a longer period of time, it is reasonable to expect a wider range of more diverse microbes to become active as the nature of the albumen is altered. The most notable effect of microbial activity on albumen is an increased acidity which produces the desirable result of more even spreading and increased gloss (Reilly 1978). Since the microbes and enzymes used in fermentation require water, the process is arrested when the albumen coated papers are left to dry.
Denaturation and fermentation are not in themselves degradation reactions. However, once denaturation and fermentation take place, the protein is more likely to participate in to the degradation reactions commonly associated with albumen prints. In the case of denaturation, the native/low energy state is altered, resulting in a more reactive protein with a higher potential energy. The fermentation process cleaves proteins by breaking peptide bonds. The result is that more amino acids are exposed and are, therefore, more likely to engage in degradation reactions.
After print manufacture, the relatively pure mix of albumen proteins are substantially modified by a numerous environmental variables. Despite these variables, there remains some characteristic qualities of aged albumen binders about which it is possible to generalize. The binder layer on virtually every surviving nineteenth century albumen photograph exhibits a characteristic cracking pattern and a yellowing noticeable in the highlights. The cracking is most likely due to the mechanical properties of the albumen becoming modified over time but this hypothesis requires further study.
The tendency for discoloration, however, has been examined and specific chemical causes have been forwarded. Such causes include, (1) reactions initiated by the sugar content of the egg white, (2) reactions initiated by the sulfur content of the egg white, (3) the tendency of proteins to bind metals (notably silver), and (4) the susceptibility of proteins to react with light. These characteristic degradation reactions are typical of the type of reactions commonly associated with proteins. All of these reactions are exacerbated by extremes of pH, temperature and relative humidity.
Maillard Browning Combination of sugar with proteins and/or amino acids is known as the Maillard reaction and has been linked to the discoloration of albumen binders (Reilly 1982). The Maillard reaction is extremely complex, variable, and difficult to characterize. What is clear, however, is that sugars not bound to the protein chain will react with the protein, forming conjugated double bonds which are chromophoric.
Sugars which have been shown to participate in Maillard browning are hexoses (notably glucose) and pentoses (notably ribose). Albumen has a free glucose content of 0.4% (Romanoff and Romanoff 1949). However, the total amount of sugar able to drive the Maillard reaction in an albumen binder layer is largely dependant on the amount and type of processing the albumen has undergone prior to coating. If a fermentation step has been employed, the sugar content of the albumen will be substantially lowered due to microorganism activity. Removing sugar in this manner been found to be effective in reducing the amount of albumen yellowing (Reilly 1982). A summary of the Maillard reaction appears below (Pellett 1982).
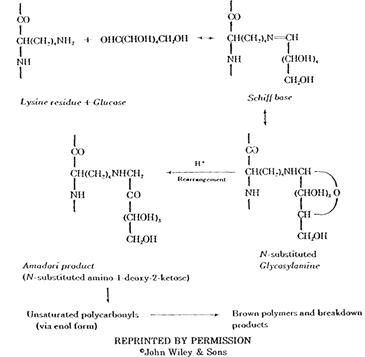
REPRINTED BY PERMISSION
©John Wiley & Sons
Sulfur As indicated by the elemental breakdown of the primary albumen proteins (see page 4), there is an appreciable amount of sulfur contained in hen's egg white. Overall, albumen contains 0.195% sulfur. Of the total sulfur content, 29.85% derives from the primary albumen protein, ovalbumin (Romanoff and Romanoff 1949). Sulfur can be chemically bound to proteins in various ways. It can exist as a sulfhydryl group (R-SH) or as a disulfide bridge (R-S-S-R). These configurations are both associated with the amino acid cysteine. The other amino acid containing sulfur is methionine (R-S-CH3). The reactivity of these groups is increased when the proteins to which they are bound are denatured (Putman 1953). In a typical degradation reaction, sulfur is oxidized by air or peroxides to form a pair of double bonds, as shown below (Creighton 1983):
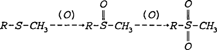
Sulfur is also known to react with the silver image material in albumen prints which causes image fading and detail loss. More relevant to the discoloration of the albumen binder, however, are reactions of sulfur with the chemically bound, non-image silver.
Silver/Protein Complexes The presence of silver in the whites of albumen prints is a phenomenon which was discovered during the latter part of the nineteenth century. It was found that this initially invisible silver, was irreversibly bound to the albumen proteins. This non-image silver (called silver albuminate or argento-albumen) could not be solubilized by the usual fixing baths of sodium thiosulfate; nor could it be removed by any other chemical means that would not destroy the image. It has been suggested that with time, this silver becomes oxidized and/or reacts with sulfur to create an overall brown/yellow staining in the highlights (Reilly 1980).
Proteins, in fact, have a great capacity to bind with metal ions. The resulting combination is known as a complex. Complexes formed with the transition metals, copper, zinc, mercury and silver, tend to be very strong chemically and can occur in conjunction with several amino acid functional groups. Silver is very strongly bound to the protein chain by sulfhydryl groups (R-SH) (Klotz 1953). The sulfhydryl groups are present associated with individual cysteine amino acids. They are also formed during denaturation and fermentation when the disulfide bridge between two cysteines is broken. The major proteins in egg white, ovalbumin, conalbumin, ovomucoid, and lysozyme, all contain significant amounts of cysteine, either alone or linked by a disulfide bond (see page 4). In addition, the albumen protein conalbumin has a notably high affinity to bind di- and trivalent metal ions. This tendency is in keeping with its biological function of sequestering metallic contaminates in the egg (Powrie 1973). Due to substantially decreased reactivity, silver ions sequestered by conalbumin would tend not to engage in degradation reactions.
The Effects of Light The absorption of ultraviolet light by proteins results in several known degradation reactions (Doty and Geidushek 1953). Types of photodecomposition include the breakdown of hydrogen bonds and the destruction of disulfide bonds (creating two reactive -SH groups). The aromatic amino acids (phenylalanine, tryptophan and tyrosine) absorb ultraviolet light and are thereby oxidized. These amino acids are all present in the egg white proteins in moderate or high proportions. Furthermore, it is known that amino acids can become separated from the protein chain by the action of light, which implies that the peptide bond itself is responsible for ultraviolet light absorption.
The main goal for individual photographers and manufacturers was to create an albumen binder with the best working properties possible. To accomplish this goal, multiple and differing processing steps were used. Based on the preceding discussion of the involved protein chemistry, it is apparent that the range of variables in processing can have a significant impact on the composition and complexity of the finished photograph. Frothing, straining, drying, combined with the other assorted processing steps, ultimately have an impact on the deterioration characteristics of individual albumen prints. The effects of display and storage environments are factors that further compound the problem. Specific methods of manufacture and past display conditions for individual photographs are usually impossible to determine. As a result, generalizations about the composition, reactions and degradation properties of the albumen binders must often be tentative and qualified. Therefore, conservation research must consider, and attempt to compensate for, the range of albumen processing techniques and the diversity of possible degradation reactions.
Creighton, T. 1983. Proteins. New York, New York: W.H. Freedman and Company.
Dickerson, R., and I. Geis. 1969. The Structure and Action of Proteins. Menlo Park, California: W.A. Benjamin Inc.
Doty, P., and P. Geidushek. 1953. Optical properties of the proteins. In The Proteins, ed. H. Neurath and K. Bailly. New York, New York: Academic Press Inc. Volume 1, Part A: 393–460.
Hardy, P. 1985. The protein amino acids. In Chemistry and Biochemistry of the Amino Acids, ed. G. C. Barrett. London; Chapman Hall Ltd. 6–24.
Klotz, I. 1953. Protein interactions. In The Proteins, ed. H. Neurath and K. Bailly. New York, New York: Academic Press Inc. Volume 1, Part B: 727–806.
Lehninger, A. 1970. Biochemistry. New York, New York: Worth Publishing.
MacDonnell, L.R., R.E. Feeney, H.L. Hanson, A. Campbell, T.F. Sugihara. 1954. The functional properties of egg white proteins. Food Technology 9: 49–53.
Nickerson, J., and L. Rosivalli. 1976. Elementary Food Science. Westport, Connecticut: AVI Publishing Co.
Pellett, P. 1982. Proteins. In The Encyclopedia of Chemical Technology. New York, New York: John Wiley & Sons Inc. 19: 314–341.
Powrie, W. 1973. Chemistry of eggs and egg products. In Egg Science and Technology, ed. W. Stadelman and O. Cotterill. Westport, Connecticut: AVI Publishing Co. 61–90.
Putman, F. 1953. Protein denaturation. In The Proteins, ed. H. Neurath and K. Bailly. New York, New York: Academic Press Inc. Volume 1, Part B: 807–892.
Romanoff, A. L. and A.J. Romanoff. 1949. The Avian Egg. New York, New York: John Wiley & Sons Inc.
Reilly, J. 1978. Manufacture and use of albumen paper. Journal of Photographic Science 26: 156–161.
Reilly, J. 1980. The Albumen and Salted Paper Book. Rochester, New York: Light Impressions Inc.
Reilly, J. 1982. Role of the Maillard or “protein sugar” reaction in highlight yellowing of albumen photographic prints. AIC Preprints, 10th Annual Meeting, American Institute for Conservation, Washington, D.C. 160–168.
Tristam, G. R. 1953. Amino acid composition of the proteins. In The Proteins, ed. H. Neurath and K. Bailly. New York, New York: Academic Press Inc. Volume 1, Part A: 181–233.
Young, G. 1963. Occurrence, classification, preparation and analysis of proteins. In Comprehensive Biochemistry, ed. M. Florkin and E. Stotz. New York, New York: Elsevier Publishing Co. Volume 7, Part 1. 1–55.
* Post-Graduate Conservation Fellow, Conservation Analytical Laboratory, Smithsonian Institution
1Ovalbumin can also be classified as a phosphoglycoprotein since phosphate and glycogen are integrated into the protein chain. However, ovalbumin is more often classified as an albumin since the phosphate prosthetic groups make up less than 5% of the total molecular weight of the protein, and since ovalbumin shares properties generally associated with the albumins.
2An important egg white protein which does not have a globular conformation is ovomucin. Ovomucin has a “fibrous” conformation which means that the polypeptide chain is not highly intertwined (as in globular proteins) but is arranged along a single axis. In nature these proteins provide structural toughness to materials such as horn, hair, and connective tissue. It is ovomucin that is responsible for the foaming of egg white when it is shaken.