Topics in Photographic Preservation 1993, Volume 5, Article 3 (pp. 14-26)
Thiourea and mineral acid silver-dip solutions were first described by Howard Brenner in 1953 for the cleaning of fragile silver objects unable withstand abrasive cleaners.1 The original formulas recommended by Brenner were modified and adapted for cleaning daguerreotypes by Mrs. Ruth K. Field, assistant curator of the Missouri Historical Society, and her formula was first published by Charles van Ravensway in 1956.2 The Missouri Historical Society formula and its variations were widely used and were thought to be perfectly safe and fool-proof treatments for daguerreotype cleaning. However, by the early 1970s some reports about problems associated with thiourea cleaners began to appear. These problems included indications that daguerreotypes could not be repeatedly cleaned in thiourea solutions as had been previously thought, that some thiourea-cleaned daguerreotypes developed milky-white coatings over time, and that some daguerreotypes developed “measles.”3 The increasing alarm over the possible adverse effects of thiourea cleaners was addressed by the fledgling Photographic Materials Group (PMG) of the American Institute for Conservation and in 1979, the membership of the PMG agreed to a moratorium on the cleaning of the daguerreotypes until more research could be done.
A few papers were published describing research to improve thiourea daguerreotype cleaning solutions,4 however, very little research was done in looking for alternatives to thiourea cleaners. Three groups published results on the use of plasmas for cleaning daguerreotypes.5 A method for electrocleaning daguerreotypes was published in 1986.6 In the latter paper, it was reported that surface characterization of daguerreotypes showed that thiourea-silver complexes remain on the surface of thiourea-cleaned daguerreotypes and that these byproducts are not removed by washing.
In 1977, Thomas M. Edmondson treated a set of eight daguerreotypes from the collection of the Torrington (Connecticut) Historical Society using the standard Missouri Historical Society formulation thiourea cleaner. The photo-documentation and reports on the treatment have been misplaced in the Historical Society's files and only Edmondson's personal notes written on the seals of the daguerreotypes are currently available. After cleaning, the daguerreotypes were resealed using Permalife paper with an adhesive made of starch paste, and sealed with a coating of B-72. The daguerreotypes were recased and subsequently stored in acid-free storage boxes in the basement of the Hotchkiss-Fyler House which is the main location of the Torrington Historical Society's collections. The house museum does not have a heating-ventilating-air conditioning (HVAC) system, however the basement storage area is a fairly stable environment with only occasional excursions of temperature or humidity during extreme conditions in the summer and winter seasons.
In December 1990, Edmondson re-examined the Torrington daguerreotypes and found that seven of the eight were showing signs of deterioration related to their previous thiourea treatment: the images were clouding over and some daguerreotypes had developed “measles.” Recognizing that the opportunity to study this set of daguerreotypes offered the possibility of verifying previously reported results on the effects of thiourea cleaners, he obtained permission to have the daguerreotypes submitted for surface analysis, re-treatment using electrocleaning, and follow-up surface analysis.
All eight daguerreotypes were submitted for surface analysis to Dr. M. Susan Barger (University of New Mexico7). The daguerreotypes were analyzed with the assistance of Dirk Kurth (University of New Mexico8) using a nitrogen purged Mattson Polaris Fourier transform infrared spectrometer with a narrow band liquid-nitrogen-cooled mercury-cadmium-telluride (MCT) detector. The polarized light from the spectrometer was incident on the sample at an angle of 75° from normal to the surface. After triangular apodization the spectral resolution was equal to 4 cm−1 or 4 wavenumbers. The moving mirror velocity was 1.264 centimeters per second and 250 to 1,000 scans were recorded for each spectra. All spectra were reported in log(R/R0), where R is the reflectivity of the sample and R0 is the reflectivity of the reference surface. Evaporated gold-on-glass slides were used as reference surfaces. In some cases, the spectra were electronically corrected in the baseline using two points at the outer edges of the bands of interest.
This experimental set-up gives spectra called reflection-absorption infrared (RAIR) spectra because the infrared beam reflected from the sample surface makes a double pass (in and out) through any absorbing surface films. The RAIR technique is used primarily to obtain spectra of thin surface films like the corrosion films found on daguerreotype surfaces. In the daguerreotype case, infrared spectroscopy is a particularly good technique for analyzing corrosion films because silver is a perfect reflector in the infrared, it therefore contributes nothing to the infrared spectra obtained from the plate surface. However, previous infrared examinations of daguerreotype surfaces merely indicated that thiourea is present on the surface of thiourea-cleaned daguerreotypes, but no other information could be derived from the earlier spectra.9 In those cases, the daguerreotype plate could only be positioned perpendicular to the infrared beam and so the pathway of the beam in the corrosion film was very short. The instrumentation available for the current examination allowed the daguerreotype to be positioned at an oblique angle to the infrared beam and thus, the beam had a longer pathway in and out of the corrosion films rusulting in an effectively thicker sample.
The spectra obtained from all eight of the untreated daguerreotypes was that of the thiourea breakdown product, dicyandiamidinsulfate. The intensity (peak height) of the spectra is indicative of the amount of dicyandiamidinsulfate present on the daguerreotype surface and this amount varied from daguerreotype to daguerreotype, however, it was not possible to determine the exact amount of breakdown product present on each daguerreotype. This is because the intensity of the spectrum for very thin films is highly dependent on both the flatness and the position of the sample. Since the daguerreotypes were not absolutely flat and there was also no way to assure that each daguerreotype was positioned in exactly the same way as the others, spectral intensity had some variation. That notwithstanding, the breakdown films appeared to be on the order of 50 to 100 nanometers thick.
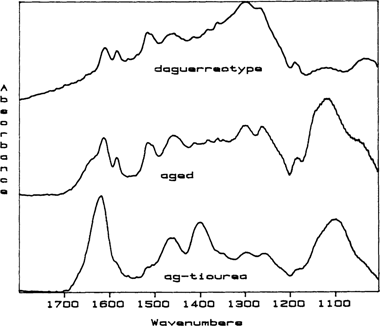
To verify the composition of any thiourea breakdown products, polished, blank daguerreotype plates were dipped in a standard thiourea cleaning solution, rinsed in water and dried in a strong stream of nitrogen gas. The spectra obtained from these experimental plates after drying was the same spectra as for a simple silver-thiourea complex. This is the initial complex formed during thiourea cleaning and it is not water soluble. These experimental plates were placed in a drying oven at 90°F in the ambient atmosphere for 4 to 5 days. RAIR spectra were taken periodically. After the aging period, the spectra obtained the surface films on the experimental plates could be superimposed with the RAIR spectra obtained from the daguerreotypes (FIGURE 1). Additionally, the infrared spectrum of a pure sample of dicyandiamidinsulfate was also compared with spectra taken from the daguerreotypes and these spectra could also be superimposed. We “recleaned” some of the experimental plates in thiourea solution and found that the second “cleaning” does not remove all of the dicyandiamidinsulfate breakdown product and that this breakdown product actually acts as a catalyst for the breakdown of any new surface films.

Figure 1 Stacked RAIR spectra for a thiourea-cleaned daguerreotype (daguerreotype), the initial spectrum for a blank daguerreotype plate dipped in thiourea cleaner (ag-thiourea), and the came plate after aging for 5 days at 90°C in ambient conditions in a drying oven (aged). The spectrum marked “ag-thiourea” shows the characteristic spectrum for the silver-thiourea complex that is initially formed on a daguerreotype plate during thiourea cleaning. The spectrum for both the daguerreotype and the artificially aged plate show the final thiourea breakdown product, dicyandiamidinsulfate. The identifiers for dicyandiamidinsulfate are the quartet of peaks located between 1400 and 1700 wavenumbers. Because these are stacked spectra, the intensities of any individual spectrum cannot be compared to the others.
Following RAIR analysis, the daguerreotypes were sent back to Edmondson who electrocleaned three of the daguerreotypes and returned them for a follow-up analysis in order to see if electrocleaning removes thiourea breakdown products. These daguerreotypes were numbers 1, 5, and 8. After electrocleaning, only number 5 was “clean” and free of surface films (FIGURE 2). This was surprising because while the silver-thiourea complex is insoluble in water, the intermediates and the final breakdown product are moderate to very soluble in water. The solubility of the intermediates and the final product, dicyandiamidinsulfate, would be altered if the corrosion film formed is a polymer instead of a monomer or if a metal salt is formed.
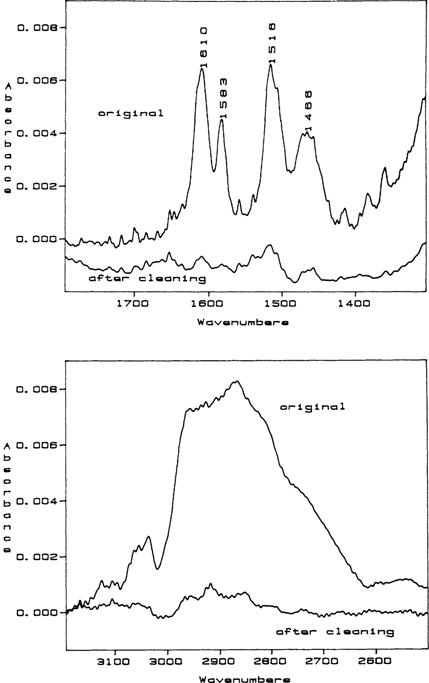
Figure 2 The initial RAIR spectrum for daguerreotype number 5 before (original) and after electrocleaning. The characteristic quartet of peaks indicative of dicyandiamidinsulfate are located between 1400 and 1700 wavenumbers. The large amorphous peak located between 2600 and 3000 wavenumbers is adventitious carbon. This is an overlay of two spectra so that their peak intensities can be compared. As can be seen from the relative heights of the two spectra, the thiourea breakdown “haze,” dicyandiamidinsulfate, has been substantially removed.
In an effort to determine if hot water would remove the dicyandiamidinsulfate, daguerreotypes 1 and 8 were dipped five and one minutes respectively in hot (200°F) deionized water and dried. These two daguerreotypes were then re-examined and in both cases the surface were found to be clean (FIGURE 3).
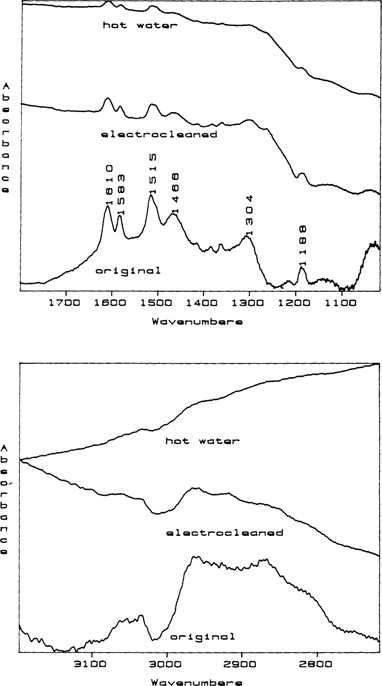
Figure 3 Stacked RAIR spectra for daguerreotype 1 before (original), after electrocleaning, and after a hot water rinse. In these stacked spectra, the intensities cannot be readily compared because the spectrometer computer fits the spectra into the frame as shown. When printed at its true intensity, the final spectrum taken after the hot water wash is essentially flat indicating that the daguerreotype surface is cleaned of dicyandiamidinsulfate.
A great deal is known about the chemistry and reactivity of thiourea with silver. However, much of this information was not considered in the application of thiourea cleaning solutions for daguerreotypes. In the development of a surface characterization tool called “Surface Enhanced Raman Spectroscopy” or SERS, silver plates dipped in thiourea solutions were used to calibrate instruments because thiourea forms a characteristic complex that can be easily detected and that cannot be removed from a silver surface by simple washing.10 Thiourea-silver complexes have been used as a photo-oxidant for one type of actinometer.11 In another context, thiourea has long been used as a stabilizing agent in photographic chemistry.12 It has been used in some monobath formulations in lieu of a fixative such as sodium thiosulfate (hypo). The primary drawback to the use of thiourea as a stabilizer is that the silver-thiourea complexes formed in these treatments degrade to silver sulfide. Additionally, in the presence of oxygen, thiourea is strongly corrosive to silver. Thus, when thiourea is used as a stabilizer for monobath processing, the resulting image eventually disappears because of the corrosive action of thiourea on silver, the formation of silver sulfide on the surface of image particles as result of the thiourea breakdown cycle and the ultimate conversion of the image to silver sulfide.13 Thiourea has also been used on silver bromide crystals to form a silver sulfide surface layer that can then be used to further adsorb certain kinds of sensitizing dyes used in making photographic emulsions.14
Thiourea (I) is known to undergo a photochemical degradation in the presence of oxygen to form cyanamid (II) and sulfuric acid.11,15 Thiourea-silver complexes undergo a similar reaction upon treatment with a base, such as ammonia.14,16 The formed cyanamid is not stable but undergoes dimerization to dicyanamid (III).17 In the presence of sulfuric acid, the dicyanamid undergoes hydrolysis forming dicyandiamidinsulfate (IV). The reaction scheme is shown below:
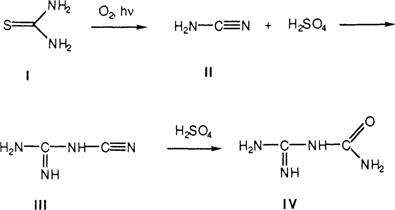
We concluded from the RAIR spectra that the decomposition of the thiourea-silver complex left on daguerreotype surfaces after cleaning is as outlined above. Basically, the chemistry of the thiourea-silver system is well known and the chemistry of the corrosion cycle of the thiourea-cleaned daguerreotype is no different from that already published in the literature. Further, re-cleaning a thiourea-cleaned daguerreotype in thiourea does not remove the corrosion breakdown products from the previous cleaning. These residual products act as catalysts to speed the degradation of new silver-thiourea complexes on the daguerreotype surface.
Lastly, when a daguerreotype is cleaned in thiourea, the thiourea corrosion cycle is initiated and it will continue until the surface is covered with dicyandiamidinsulfate. The rate of the corrosion cycle is dependent upon many factors including storage conditions, the effectiveness of the daguerreotype seal, and whether or not the daguerreotype is displayed. The corrosion products found on a thiourea-cleaned daguerreotype are not limited to the thiourea breakdown products, but they also include silver phosphate, silver oxides, and sulfuric acid. Thus, attempting to remove the thiourea breakdown products using a hot water wash will have limited effect. A daguerreotype needs to be treated in a way that will remove all of the corrosion products no matter what the source.
This project underscores how important it is for conservators to revaluate treatments and their application. It is imperative that conservators keep an open mind and that they be willing to examine not only their procedures and materials, but also their philosophical perspective towards their work. Thus, it is important that conservators be certain that treatments are as appropriate and safe for objects as is possible to determine, and it is equally as important that the conservator be clear about their objectives in treatment.
The object of this particular project was to establish that the use of thiourea cleaners for daguerreotypes presents real dangers to these objects. It was also hoped that electrocleaning would be shown to be a single, effective method to remove thiourea corrosion films from daguerreotype surfaces. The results show that the thiourea corrosion cycle is clearly a factor in the degradation of daguerreotypes previously cleaned in thiouorea silver dips. On the other hand, electrocleaning was effective in removing almost all of the thiourea corrosion products. In some cases, a barely detectable amount of thiourea breakdown products were left on the daguerreotype plates after electrocleaning. In all cases, a hot water rinse removed any trace amounts of thiourea breakdown products following electrocleaning. The daguerreotype cleaning problem is very complex and must be considered carefully. A conservator must decide on a course of action that is appropriate for each case. This means that it might not be appropriate to rely on a standard method that will be good in the general case and not be applicable in a specific situation.
Thiourea cleaning treatments were once considered safe, effective, and accepted procedures for the care of daguerreotypes. No finger of blame can be pointed at those who used this procedure before the Photographic Materials Group's moratorium on its use. Any uninformed dealer or collector today may be excused for its use, but they do need to be educated about the damage that this treatment inflicts on daguerreotypes. However, for any person who has been aware both of the concerns about this treatment that have been raised in many articles and presentations over the last ten years, there can be no excuse for the continued use of thiourea cleaners. Any doubts as to the damage caused by these treatments should now be erased, and it should be clear that it is no longer acceptable under any circumstances to continue to use thiourea treatments or to suggest their use. The information presented here clearly shows that thiourea treatments cause irreparable damage to allowing the daguerreotypes and that there are no circumstances when this treatment can be considered ethical.
We wish to thank Mark McEachern and the Torrington (Connecticut) Historical Society for giving permission for the analysis and retreatment of their daguerreotypes as described here. We also wish to thank Dirk Kurth for his role in carrying out the analyses and Dr. Thomas Bein (now, Department of Chemistry, Purdue University) for making it possible to use one of the spectrometers in his group.
1. Howard Brenner, “Silver Dips,” Soap and Sanitary Chemicals 29 (1953): 161–167, 183.
2. Charles van Ravensway, “An Improved Method for the Restoration of Daguerreotypes, Image 5 (1956): 156–159.
The Missouri Historical Society formula is as follows:
Distilled water |
500 cubic centimeters |
Thiourea |
70 grams |
Phosphoric acid (85%) |
80 cc |
Non-ionic wetting agent (e.g Kodak “Photo-Flo” solution) |
2 cc |
Distilled water to make |
1,000 cc |
The daguerreotype is uncased and washed in distilled water to remove any surface dirt. It is then immersed in the thiourea solution until any discoloration is washed away. Finally, the plate is rinsed in tap water, possibly washed in distilled or soapy water, rinsed again in water. A final alcohol dip is sometimes used to facilitate drying of the plate.
3. Leon Jacobson and W. E. Leyshon, “The Daguerreian Measles Mystery,” Graphic Antiquarian (Spring 1974): 14–15.
4. For example see: T.J. Collings and F.J. Young, “Improvements in Some Tests and Techniques in Photograph Conservation,” Studies in Conservation 21 (1976): 79–84; Siegfried Rempel, “Recent Investigations on the Cleaning of Daguerreotypes,” in AIC Preprints (Washington, DC: American Institute for Conservation, 1980): 99–105; Alice Swan, “The Preservation of Daguerreotypes,” in AIC Preprints (Washington, DC: American Institute for Conservation, 1981): 164–172.
5. Vincent Daniels, “Plasma Reduction of Silver Tarnish on Daguerreotypes,” Studies in Conservation 26 (1981): 45–49; M. Susan Barger, S.V. Krishnaswamy, and R. Messier, “The Cleaning of Daguerreotypes: I. Physical Sputter Cleaning. A New Technique,” in AIC Preprints (Washington, DC, 1982): 9–20; Barger, Krishnaswamy, and Messier, “The Cleaning of Daguerreotypes: Comparison of Cleaning Methods,” Journal of the American Institute for Conservation 22 (1982): 13–24; Mogens S. Koch and Anker Sjøgren, “Behandlung von Daguerreotypien mit Wasserstoffplasma,” Maltechnik Restauro 90 (1984): 58–64.
6. M. Susan Barger, A.P. Giri, William B. White, and Thomas M. Edmondson, “The Cleaning of Daguerreotypes,” Studies in Conservation 31 (1986): 15–28.
7. Associate Adjunct Professor, Department of Geology; on leave: Associate Research Professor, Department of Materials Science and Engineering, Johns Hopkins University, Baltimore, MD.
8. Graduate Student, Department of Chemistry; now Doctoral Candidate, Department of Chemistry, Purdue University, West Lafayette, IN.
9. See FIGURE 10.5 in M. Susan Barger and William B. White, The Daguerreotype: Nineteenth Century Technology and American Science (Washington, DC: Smithsonian Institution Press, 1991): 168.
10. B.H. Loo, “Molecular Orientation of Thiourea Chemisorbed on Copper and Silver Surfaces,” Chemical Physics Letters 89 (1982): 346–350.
11. Otto Warburg and Victor Schoken, “A Monometric Actinometer for the Visible Spectrum,” Archive of Biochemistry 21 (1949): 363–369.
12. Rolf S. Bruenner, “Thiourea and Its Derivatives in Photographic Stabilization Processing,” Photographic Science and Engineering 4 (1960): 186–195.
13. See: G.I.P. Levenson and K.H. Stephen in The Theory of the Photographic Process, 4th edition, (ed. T.H. James, J.F. Hamilton, G.C. Higgins, and J.E. Starr) New York: Macmillan Publishing Co., Inc., 1977, Chapter 15.
14. T.H. James and W. Vaneslow, “Kinetics of the Reaction Between Silver Bromide and An Adsorbed Layer of Allylthiourea,” Journal of Physical Chemistry 57 (1953): 725–729.
15. G.O. Schenck and H. Wirth, Naturwissenschaften, Teil B 40 (1953): 141.
16. W.I. Stephen and A. Townshend, “The Reaction of Silver (I) Ions with Organic Compounds Containing HN-C=S Grouping. Part II. Some thiourea Derivatives,” Journal of the American Chemical Society (A) (1966): 166–168.
17. J. Söll and A. Stutzer, “Mitteilungen uber einige neue Verbindungen, die aus Guanylharnstoff, die aus Guanylharnstoff und aus Diguanid erhalten wurden,” Ber. dt. Chem. Ges. 42 (1910): 4532–4541.
1 To whom correspondence should be addressed.
2 On leave from: Department of Materials Science and Engineering, Johns Hopkins University, Baltimorel, MD 21218.