Topics in Photographic Preservation 1993, Volume 5, Article 10 (pp. 89-94)
The focus of this paper will be a description of the exhibition of a photogenic drawing which was made by William Henry Fox Talbot around 1835. Because they are important to the discussion of this image, Talbot's early experiments in photography will first be described.
Although Talbot announced his invention of photography to the Royal Society in January 1839, his experiments began in 1834. On a trip to Lake Como in 1833, Talbot had been frustrated by his inability to record the scenery with a camera lucida. He later wrote in 1844 in the Pencil of Nature that he found that “the faithless pencil had only left traces on the paper melancholy to behold.”1 Earlier attempts at drawing with a camera obscura had also been unsatisfactory, and Talbot began to wish for a method by which images would “imprint themselves durably, and remain fixed upon the paper!”2 When Talbot returned from his continental tour to his home at Lacock Abbey in 1834, he began his experiments.
Initially, Talbot coated drawing paper with a solution of sodium chloride, and brushed silver nitrate on its dried surface. He then placed a specimen on this sensitized paper and, once secured, exposed it to sunlight. When the paper grew dark, as the silver salts were reduced to metallic silver by the action of the sun, the specimen was removed, leaving its impression on the paper.
Talbot knew that images made by this method would darken over time and sought to prevent this by the application of stabilizing solutions, notably potassium iodide and sodium chloride, which converted the remaining unexposed silver salts to a less sensitive form. He was not yet using the sodium thiosulfate fixer, or hypo, which later became standard. Properly used, sodium thiosulfate will remove unexposed silver salts from the paper support, producing an image which is much less sensitive to light than those images which have been stabilized with salt solutions.
[For the purposes of clarity in this discussion, only sodium thiosulfate has been referred to as fixer, while salt solutions have been called stabilizers. However, it is important to note that the distinction in nomenclature is a modern one and should not be understood as the terminology that Talbot himself used, which is substantially different than the one chosen by the author for this paper.]
In a 1839 letter which was read before the Royal Society, Talbot discussed his early attempts to stabilize photographs. He wrote, “After having tried ammonia, and several other agents, with imperfect success, the first thing which gave me a successful result was the iodide of potassium, much diluted with water.”3 In the same letter, Talbot stated that images stabilized in this way were primrose, meaning yellow, colored,4 and he cautioned that the potassium iodide solution must be carefully prepared, or it would cause image fading. The actual discovery of the usefulness of potassium iodide was apparently made near the end of 1834.5
Talbot subsequently discovered the stabilizing property of sodium chloride early in 1835, while searching for a sensitizer.6 He observed that the most sensitive parts of the paper were the edges and recognized that these areas would have absorbed less of the salt during its application in the sensitization step. So he correctly surmised that if less salt promoted greater light sensitivity, more salt would lessen sensitivity. Talbot experimented with other stabilizers, but ultimately decided that a saturated solution of sodium chloride was as reliable and simple as any of the others. He also observed that images stabilized by sodium chloride might eventually “colour themselves of a pale lilac tint”7 in highlight areas.
In spite of the impressive scope of Talbot's scientific knowledge, he apparently was not aware that sodium thiosulfate was a solvent for certain silver salts until he was introduced to it by Sir John Herschel in February 1839.8 While Talbot immediately began to explore the usefulness of sodium thiosulfate, he also continued his investigations with salt solutions and other reagents meant to deter darkening.9 Therefore, while we may assume that the photographs that Talbot made before 1839 were stabilized with salt or other experimental means, it is important to recognize that photographs made after February 1839 were not inevitably fixed with sodium thiosulfate.
Because Talbot continued to experiment with photographic chemistry well after his introduction to sodium thiosulfate, it is difficult to determine the light sensitivity of individual images. In addition to original processing, there are other factors, such as conditions storage, previous use, and subsequent restoration treatments, which effect the stability and the appearance of Talbot's early photographs.
Following is an account of the J. Paul Getty Museum's experience exhibiting a photogenic drawing entitled Linen Textile Fragment. This image is believed to have been made by Talbot in 1835. The author was not employed by the museum when this object was displayed in 1989; therefore, this information was reconstructed from files at the Department of Photographs at the J. Paul Getty Museum and with the input of departmental staff members who were involved with the exhibition.
Linen Textile Fragment was made by Talbot's early process, described above. That is, a piece of paper was first coated with a solution of sodium chloride, followed by a solution of silver nitrate. After exposure, the photogenic drawing was presumably stabilized with a solution of sodium chloride, which would have converted unexposed silver salts to a form which was less sensitive, but not stable, to light. While in the past there has not been unanimous agreement about what kind of salt was used for stabilization, we are certain that the photogenic drawing was not fixed with sodium thiosulfate, which would have removed unexposed silver salts, producing a more light stable image. The tentative identification of the sensitizing and stabilizing solutions was provided by scholars, based on their experience with Talbot's work and the color and tonality of the image.10 To the author's knowledge, there are no specific technical references in the literature of art history or conservation which describe this image.
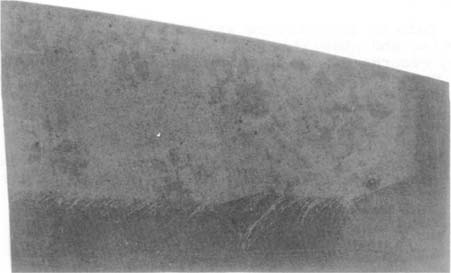
Before exhibition, the highlights of this photogenic drawing had a brownish pink tonality. During exhibition, they began to print out, that is, to darken. This printing-out occurred because the salt solution which was used to stabilize the image did not completely render the unexposed silver salts insensitive to light. Subsequent exposure to light caused these residual silver salts to be reduced to metallic silver, resulting in increased density to the image.
The photogenic drawing now appears darker and redder in the highlight areas. It cannot be positively stated that it has also darkened in the border areas, which were already quite dense. Uneven mottling has occurred in the image areas, obscuring the image in some places, especially around its edges. Although the subject is still visible, the photogenic drawing is considerably darker overall, and much of the detail has been lost. Given the current state of conservation research, these changes may be described as irreversible at this time.
Unfortunately, no densitometric readings were made before this image was displayed, so quantitative information about the color change is not available. In the absence of densitometric data, color change must be described in subjective terms; however, such change has definitely occurred, and the empirical evidence is compelling. It is not the author's intention to minimize the importance of quantitative data, but to state that, in this case, data would also serve to reinforce what can easily be seen.
The image was observed to have printed out after about five weeks of exhibition in the photographs gallery of the J. Paul Getty Museum11, but it cannot be stated definitively when darkening began or how quickly it progressed. The gallery generally maintains a temperature of 70F +/− 2F and a relative humidity of 50% +/−5%.
The photogenic drawing was displayed, framed under UF4 Plexiglas, at approximately 5 foot-candles, and the illumination used was tungsten incandescent with UV filtration. It is estimated that the framed object was exposed to 5 foot-candles of illumination, 8 hours per day, for 35 days12. No viewer operated curtain was used.
This regrettable incident has caused the J. Paul Getty Museum to adopt a more conservative exhibition policy for experimental photographs. As a conservator, it is the author's opinion that these images should not be put at unnecessary risk, and that facsimiles can serve for the purposes of exhibition. However, the pressure to show fragile photographs may sometimes be strong, as the curatorial responsibility to share the collection occasionally conflicts with the responsibility to preserve it. This is a topic which continues to be the focus of discussion, and at times dissension, between conservators and curators. It is hoped that knowledge of the J. Paul Getty Museum's experience will be useful to this ongoing dialogue.
William Henry Fox Talbot, Linen Textile Fragment, ca. 1835, photogenic drawing before exhibition

William Henry Fox Talbot, Linen Textile Fragment, circa 1835, photogenic drawing after approximately five weeks of exhibition at 5 foot-candles.

The author wishes to acknowledge the input of Ernie Mack and Gordon Baldwin of the Department of Photographs at the J. Paul Getty Museum.
1. William Henry Fox Talbot, The Pencil of Nature (London: Longman, Brown, Green, and Longmans, 1844). Talbot made this comment in the unpaginated preface, “Brief Historical Sketch of the Invention of the Art”.
2. Talbot, Pencil of Nature, preface.
3. Talbot, “An Account of the Processes employed in Photogenic Drawing, in a Letter to Samuel H. Christie, Esq. Sec. R. S., from H. Talbot. Esq., F. R. S.” [reprinted in Gail Buckland, Fox Talbot and the Invention of Photography (Boston: David R. Godine, 1980), p. 48]. This was read before the Royal Society, February 21, 1839 and subsequently published in Philosophical Magazine 14, no. 88 (March 1839).
4. Talbot, letter to Christie.
5. Talbot, Pencil of Nature, preface.
6. Talbot, notebook on deposit at the Fox Talbot Museum, Lacock, [reprinted in Buckland, p. 29]. This specific section is undated, but directly follows entries for January 1835. Talbot also discussed this in Pencil of Nature, preface.
7. Talbot, letter to Christie.
8. Sir John Herschel, notebook at the Science Museum Library, London, [reprinted in Larry J. Schaaf, Out of the Shadows: Herschel, Talbot and the Invention of Photography (New Haven: Yale University Press, 1992), p. 50]. Herschel, when Talbot visited him on February 1, 1839, fixed half of a photogenic drawing with sodium thiosulfate and preserved both of the pieces in his notebook.
9. Talbot, notebook in the Herschel Collection, Royal Society, London, [reprinted in Buckland, p. 40–59]. Talbot's notebooks and correspondence discuss his continuing experiments with sodium thiosulfate and stabilizers.
10. Date of manufacture and notes on original processing (i. e. salting and stabilizing solutions) are contained in the cataloguing records of the Department of Photographs at the J. Paul Getty Museum.
11. Ernie Mack (Department of Photographs, J. Paul Getty Museum), personal communication with the author, January 1993.
12. Ernie Mack (Department of Photographs, J. Paul Getty Museum), personal communication with the author, January 1993.