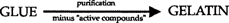
Topics in Photographic Preservation 1993, Volume 5, Article 15 (pp. 146-150)
Photographs in albums from the 19th century often exhibit fading along the edges. Sometimes, the fading, i.e., areas with a loss in image density, is more pronounced along the three edges of a page facing outwards; in such a case it is less along the edge adjacent to the spine of the album. In other cases, the fading is conspicuous as a narrow bleached band along all four edges. This can be observed in photographic prints dating from the first decade of photography. We have studied the possible causes for this pronounced fading all around the edges of a print, and present evidence that it was most likely caused by the adhesive that was used for mounting the print. Our observations confirm that the inherent stability of the image silver towards chemical oxidation, in various types of photographs, depends in a consistent manner upon their structure and the respective image silver morphology.
Photographs on paper made during the first ten years or so of photography often exhibit a narrow area of fading along the picture's edges. This faded band varies in width from a few millimeters to a width of one to two centimeters. Prominent examples of edge fading, as we will call this occurrence in the course of this article, include positive prints by H.W.F. Talbot, D.O. Hill and R. Adamson, and J.M Cameron. We have examined possible causes for this pronounced fading observed along the edges of early photographs.
It is obvious to speculate at once on the possibility that an adhesive, used to attach the photograph to a support, may have caused the observed discoloration. This was indeed our starting hypothesis. We observed that during the first decade of photography, i.e., from 1839 to 1850, no journal dedicated to photography existed. We also noted that in photographs taken after about 1850, the occurrence of a pronounced edge fading appears to decrease markedly. Since we do not have the opportunity to consult general journals from that period, (such as, for example, Dingler's Polytechnical Journal), we do not know whether there were recommendations during that time for the use of certain adhesives for the mounting of photographs. It would not be surprising, however, to eventually find an early literature reference with a recommendation not to use some adhesives.
At any rate, our assumption was that early photographers had no experience of various factors that might affect the chemical stability of the silver image. Such factors may include mounting adhesives. Before the arrival of modern synthetic adhesives, they were either of plant or of animal origin. The three languages English, French, and German each have two different terms for the two natural adhesives: while glue is of animal origin, paste indicates plant origin, as in rice starch paste, or wheat starch paste. [In French, the corresponding terms are colle forte and pâte; in German they are (tierischer) Leim and Kleister.] We estimated that glues of animal origin might contain substances aggressive enough to react with image silver to form silver salts and so cause visible discoloration.
We prepared a number of strips of photographic prints with a grey scale on salted paper, albumen paper, Eastman Kodak Studio Proof (a gelatin printing-out paper), Eastman Kodak Azo (a contact-speed paper), and Ilford Galerie (a typical projection-speed enlarging paper). Two different commercially available glues1 and dilute solutions of pure photographic gelatin were brushed on the back of the prints in various patterns. While some prints were kept at ambient conditions in the laboratory, others were exposed to a temperature of 60°C and 70% relative humidity for seven days. Salted paper prints developed strong discoloration after two hours at accelerated ageing conditions, and after 24 hours at ambient conditions (20–23°C/55%RH). Similar discolorations were observed, but at a slower rate of formation, in the albumen prints, followed by the Studio Proof, Azo, and bromide papers. Pure photographic gelatin (0.5%, 1%, 2% solutions in warm water) showed no ability to cause discoloration.
In seeking to re-create the edge fading seen on well-known photographs, we copied (by courtesy of the curator of the photography department at the National Gallery of Canada) two prints from the same negative by D.O. Hill and R. Adamson, a portrait of Rev. Jones. One was a salted paper print believed to be dating from about 1845, and the other a carbon print made from the original paper negative in 1916 by Jessie Bertram. While the carbon print shows no fading or discoloration, the salt print exhibits strong edge fading all around the image. Since the format of both prints was about 6 1/2 by 8 1/2 inches, we photographed them on 8×10″ sheet film [Kodak Professional Copy Film 4125] in a 1:1 ratio. This allowed us to obtain a copy negative from the original carbon print, which was exposed to salted paper prepared in our laboratory to produce sample prints. The sample prints had the same format as the original. One of the more active glues (Liquid Hide Glue) was brushed on the back of a sample print along the edges, so as to simulate the application of an adhesive to mount the photograph onto an album page. The glue, as had been shown earlier on strips of grey scales, caused discoloration along the edges in a pattern resembling that observed on the original print from about 1845.
These observations confirm the recommendation made in 1858 by Colin Sinclair in the Journal of the Photographic Society [No. 67; June 21, 1858]. In that article, a wide variety of adhesives, mostly pastes of plant origin, are discussed for the mounting of photographs. Among glues of animal origin, Isinglass and albumen were found to be poor adhesives, the latter being capable of causing yellowing of prints. Other studies published shortly after 1870 evaluated fresh starch paste to be very stable, and called it ideal for the mounting of photographs. Pure gelatin with an alcohol solution was found to be the best adhesive. It will keep indefinitely and will not sour.
We suspect that some time during the first ten to fifteen years of photography, the knowledge spread quickly that glues are unsuitable for the mounting of photographs. Perhaps it was published in a non-photographic journal in a reference yet to be found, but perhaps it was spread by word of mouth.
Since glue and gelatin are made from the same raw materials of animal origin, one might consider gelatin as a refined glue derivative. Both have adhesive properties, but gelatin is obviously photographically inert since it is the predominant material for embedding silver halides in the raw stock and the subsequently formed image silver in contemporary silver gelatin photographic technology. Therefore, glue contains an aggressive substance capable of reacting with image silver that is no longer present in gelatin:
We set out to identify the active ingredient in animal glue. By studying the known composition of various glues and of gelatins, several substances in glue were suspected of being reactive ingredients, among them sulfur dioxide, hydrogen peroxide, thioalcohols, and sulfur bound in cystine. One compound was conspicuous in containing an active sulfur atom (characterized as a “thio” sulfur) that can react with image silver: thiourea, CS(NH2)2. We prepared solutions of thiourea in 0.1%, 1%, and 5% concentration in water, and applied the solutions to the back of the prints described earlier. In all cases, discoloration of the print occurred at the same rate and in a similar pattern as observed earlier for the effect of two glues. We therefore suggest that the pronounced discoloration along the edges of salted paper prints made during the first ten years of photography was caused by the glue used to mount them onto book or album pages. The specific active ingredient in the glue was probably thiourea.
We have presented, however, only indirect evidence for our conclusions. If two compounds A and B react chemically to form a third compound, and if that reaction goes to completion, then either of the two initial reactants have disappeared and so cannot be shown to be present in the system anymore:
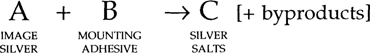
If B is consumed in this reaction, it cannot be shown to be present now.
It would therefore be fruitless to search for the presence of unreacted thiourea on the back of prints showing strong edge fading. Any amounts would be too small to detect with certainty, but whatever amount there was originally, it will have reacted with the available image silver.
Our observations also gave us further confirmation of the different reactivity of various print materials according to their structure and the morphology of the image-forming silver grains. The stability of processed black-and-white photographic materials is strongly affected by oxidizing chemicals, such as thiourea, in the presence of moisture and heat. They can be arranged in order of their inherent stability, which is determined by their resistance toward the effect of oxidizing chemicals. That resistance increases in the following order: salted paper prints < albumen prints < silver gelatin printing-out papers < chloride paper < bromide paper (< negative films). The image silver in salted paper prints is the most reactive for several reasons: firstly, the image-forming silver grains in these prints are smaller than in developed-out papers by a factor of approximately 100. Therefore, they are much more sensitive toward reactive materials in comparison to developed-out prints. Secondly, the lack of a separate binding layer (termed the emulsion layer in contemporary print materials) only reinforces this sensitivity, since the paper support is quite permeable for many oxidizing gases, such as hydrogen peroxide. The absence of such a protective layer further increases the danger of mechanical damage by abrasion. [Consequently, modern films and papers carry a separate layer as protection against abrasion coated on top of the image layer]. And finally, early photographs on salted paper were generally made using support materials of unknown quality with the concurrent uncertainty regarding their stability. Albumen prints, as the name indicates, carry the image particle inside a protective layer derived from the white of eggs. This results in increased image stability. Silver gelatin printing-out papers generally have the additional benefit of a baryta layer that is sandwiched between the paper support and the image layer, which protects the image from impurities migrating through the paper support. Chloride papers and bromide papers are both developed-out prints with silver particles embedded in a gelatin layer: the silver grains are larger, and therefore chemically more resistant than the grains in salt prints and albumen prints. They also have a baryta layer with the benefits noted above. Photographic films have traditionally had a requirement for high light sensitivity, which results in a comparatively coarse image structure and increased resistance to chemical attack.
* National Archives of Canada, Ottawa, Ontario, Canada
1 Franklin Chemicals Liquid Hide Glue; Lepage Canada Strength Liquid Glue