
Topics in Photographic Preservation 1999, Volume 8, Article 8 (pp. 56-59)

Figure 1
Roger Parry,
Hand Holding Insect, cellulose nitrate negative, 7″ × 9″,
SFMOMA 84.921
Watermark impression on cellulose nitrate negative, normal light, front view
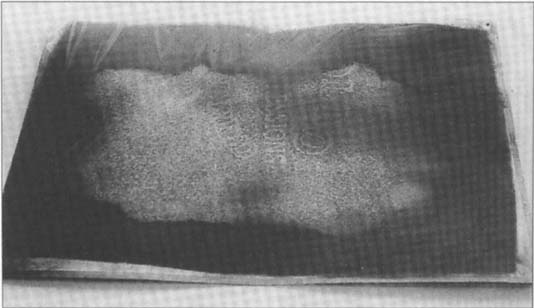
Figure 2
Roger Parry, Hand Holding Insect, cellulose nitrate negative, 7″ × 9″, SFMOMA 84.921
Watermark impression on cellulose nitrate negative, normal light, oblique view
This paper concerns a phenomenon which occurred during the re-housing of a collection of cellulose nitrate negatives at the San Francisco Museum of Modern Art, in which silver mirroring already present on the surface of the deteriorated negatives changed to form the pattern or “letters” of the watermark on the paper envelopes used for their housing. This phenomenon occurred in less than two months.
SFMOMA owns a collection of approximately 1000 original cellulose nitrate negatives taken by the French Surrealist photographer Roger Parry in the 1920's - 1930's. The negatives were examined during a survey of the photography collection, approximately 5 years ago. It was discovered that although the majority of them were generally in good condition, a number of them were undergoing visible deterioration.
Five stages of deterioration of cellulose nitrate have been established to track its decomposition:
The majority of the Parry negatives in our collection exhibit the signs of the first and second stages of deterioration, with amber discoloration, image fading, silver mirroring and slightly tacky emulsion. There are 10–15 negatives which are in the fourth and fifth stages of deterioration.
A project was recently undertaken at SFMOMA to duplicate the negatives (except for the Stage Four and Stage Five examples) so that the originals could be placed in cold storage and would no longer need to be accessed. The collection was sent to the Chicago Albumen Works in Housatonic, MA, where each negative was contact printed to create an interpositive and a subsequent negative was produced.
Once the negatives had been duplicated, a storage plan was conceived. The deterioration of cellulose nitrate in normal storage conditions is compounded by the fact that the material is auto-catalytic—the evolved gases accelerate the decomposition of the film. Therefore, the halting of deterioration was critical to the preservation of our collection of original negatives. It was decided to place the negatives in cold storage in a freezer. The low temperature would significantly slow, and perhaps stop, the deterioration rate of the cellulose nitrate material.
It is also important to decrease the relative humidity in the storage environment. This would be accomplished by conditioning the negatives to 30–40% RH, and then double-sealing them in vapor-proof laminated aluminum-foil bags2. This would prevent condensation upon placement in freezer storage. An additional paper moisture buffer would be provided by re-housing the negatives in individual paper enclosures, and placing a stack of the sleeved negatives in an archival storage box. This box would then be sealed in the vapor seal bags.
This cold storage plan is in keeping with the practices of many institutions at this time, which realize the importance of preserving original nitrate negatives with important artifact value3. The idea that all cellulose nitrate material should be immediately disposed of is changing as institutions realize that the deterioration and risk of ignition of cellulose nitrate is basically halted in cold storage.
The first steps of the rehousing project involved placing each negative in a paper envelope. The envelopes chosen were Apollo Balanced Seam Envelopes from Light Impressions. Apollo envelopes are buffered to pH 8.0–8.5 with 2% calcium carbonate. A stack of negatives was then placed inside Buffered Drop-Front Boxes from Conservation Materials. Once rehoused, the boxes of negatives remained in our climate-controlled conservation studio.
The second step of sealing the negatives in vapor-seal envelopes and freezing them was postponed for two months while waiting for identification labels, which were to be placed on each negative envelope. When the labels arrived, attention was once again focused on the storage project. In examining the negatives, it was noticed that approximately ten of the Stage One deteriorated negatives now had the words of the watermark (“Apollo Buffered, Light Impressions”) “written” on them in silver mirroring. (See illustrations, Figs. 1 and 2) In most cases, the mirroring was greater in the letters of the watermark, in a few it was the opposite. It had been noted, upon initial examination two months prior, that a general pattern of overall silver mirroring concentrated in the center of each negative was already present on many of the negatives.
Once this problem was discovered, other photograph conservators and conservation scientists were contacted, and a posting was written to the Conservation DistList4. Dennis Inch was also contacted (Vice President of Light Impressions, at that time). No one we spoke with had observed this phenomenon before except for Giovanna Di Peitro, a conservation science student in Basel, Switzerland. She had been studying silver mirroring on gelatin glass plates which were stored in glassine envelopes. There was a pattern of mirroring on a number of plates which corresponded to the creases in the glassine envelopes each was stored in. Her observations were similar to those made about the SFMOMA examples, in that sometimes the mirroring corresponded to the “raised” areas and sometimes to the lower parts of the creases.
Many questions were raised in the discussions that followed. We believe we were able to rule out the possibility that the Light Impressions watermark was a chemical watermark which effected the negatives, for we were assured by Dennis Inch that the watermarking was not chemical. This was strengthened by Doug Nishimura of IPI who also contacted Mr. Inch. Mr. Nishimura was confident that Mr. Inch was very familiar with the materials and their production. Additionally, both of the Light Impressions interleaving materials, Apollo & Renaissance, have passed the Photographic Activity Test and also meet ANSI standards.
In a discussion with Doug Nishimura, he said that he believed that the most likely cause of the silver mirror “writing” was that the negative was continuing to out-gas nitrogen oxides, causing further oxidation and migration of the silver to form more mirroring. This corresponds to a fundamental quality of cellulose-nitrate deterioration—the autocatalytic nature of the reaction.
If we look at the watermark and think of the minuscule yet very real difference in its physical topography, we can begin to form one possible explanation. In the area of the watermark, the paper is thinner and therefore slightly raised above the surface of the rest of the paper envelope. In these areas, the paper is cleanly not in contact with the negative. Areas in contact may be neutralizing enough of the most aggressive nitrogen oxides being emitted from the cellulose nitrate. Nitrogen dioxides, for example are both strongly oxidizing and very acidic, and are known to effect the silver image5. In the area of the watermark where the paper is not in contact, and therefore not neutralizing the nitrogen oxides, there is a greater amount of mirroring occurring6.
This explanation seems possible for the areas in which the mirrored “writing” is greater than in the surrounding areas, but does not explain the opposite phenomenon, in which the mirroring in the letters is less than in the surrounding areas.
It does seem likely that in housing the negatives in paper enclosures and filling up each archival box with a stack of the negatives approximately 3″ high, a micro-climate had been set-up in which gases emitted from the negatives were trapped, creating a high pollutant concentration. This environment seems to have sped up the deterioration of the negatives, to the point that a dramatic visible change occurred in only two months.
Furthermore, there obviously is a correlation between the watermark and the silver mirror “writing” which had occurred on the surface of the negatives. Perhaps the highest concentration of gases was in the small spaces created by the topography of the watermark. Another possibility is that the nitrogen oxides could move more easily through the thinner areas of the paper, reacting with the silver image material in these areas only.
We realize that whatever the reaction that is occurring and the reason for it, the process of freezing the negatives will most likely halt this deterioration, as well as the general deterioration of the cellulose nitrate. However, we thought that it was important to notify the conservation community about the phenomenon. We raise the question of whether a similar phenomenon is occurring in museum and library collections which are storing cellulose nitrate negatives in non-cold storage environments. It may be that the problem has not been identified in these collections yet because the negatives are accessed infrequently. We hope that there will be questions and ideas generated from this paper which will help us to understand the occurrence, and alert others to keep an eye out for it in their own collections.
1The five stages of the decomposition of cellulose nitrate film were published in an article by J. W. Cummings, A.C. Hutton, and H. Silfis in the March 1950 issue of the Journal of the Society of Motion Picture and Television Engineers.
2from Henry Wilhelm, The Permanence and Care of Color Photographs, Preservation Publishing Co., Grinnell, Iowa, 1993, chapter 19.
3These institutions include: The Historic New Orleans Collection in New Orleans, Louisiana; the Center for Creative Photography, at the University of Arizona in Tucson, Arizona; the Humanities Research Center at the University of Texas at Austin; the California Historical Society, in San Francisco, California.
4The Conservation DistList is connected to the Conservation Online website (COOL), created by Walter Henry at the Stanford University Preservation Department.
5Carroll, J.F. & Calhoun, J.M., “Effect of Nitrogen Oxide Gases On Processed Acetate Film”, 1955, J.SMPTE, 64, 501–507
6Besides nitrogen dioxide, the other nitrogen oxides that are emitted from nitrate film are nitrous oxide and nitric oxide.