Topics in Photographic Preservation 2001, Volume 9, Article 1 (pp. 1-43)
The Chemical Treatment of Photographic Materials Workshop was held in Kent, Connecticut at Jose Orraca's studio, September 23 – 25, 1999. Treatment methods were divided into four groups: Chemical Intensification, Silver Mirroring, Reduction/Intensification, and Yellowing/Stain Reduction. Ten treatments were studied in total.
1. Chemical Intensification
2. Silver Mirroring
3. Reduction/Intensification
4. Yellowing/Stain Reduction
Observations were discussed following the workshop, and it was concluded that three processes in particular showed promise for use in conservation: C. Fischer Formula, iodine/alcohol, and sodium borohydride. These conclusions were based only on visual observations, and no property measurements were taken. Sodium borohydride has been thoroughly discussed by Baas et al.1,2 and will not be discussed in any detail here. However, it and the other seven processes will be briefly described below before a detailed discussion of C. Fischer Formula and iodine/alcohol.
Mogens Koch has kindly provided instructions for the variations of iodine/alcohol and bleach and redevelopment that they teach at the School of Conservation in Denmark. These instructions are provided in appendix A.
The theory behind bleach and redevelopment is that all silver compounds are converted into silver halide by the use of a reasonably strong bleach and an excess of halide. The silver halide is then exposed to light and reprocessed. It is important that the developer does not contain a large amount of silver solvent such as sulfite in order to prevent erosion of the image. The silver solvent will tend to solubilize the redeveloping silver filament in order to keep the grain size down but, in the process, will reduce the amount of redeveloped image silver.
This process can be successful as long as the original image silver particles are reasonably intact. The large image silver particles are required for the silver to build around. If little remains of the original silver filaments, the silver halide redevelops as fine colloidal silver scattered in the emulsion and the visual effect is that the image bleaches but doesn't redevelop. This requirement limits the treatment to DOP, since the photolytic silver in POPs is colloidal.
Silver that has migrated too far from the original image particles will bleach and redevelop where it is. Therefore, silver mirroring will visually remain unaffected. Very small discrete colloidal silver particles may also dissolve during fixing, removing some stain but resulting in silver loss.
This process is often referred to as an intensification method, although not all suggested bleaches result in the addition of silver or other chemicals into the image. Therefore, it is not necessarily an intensification process.
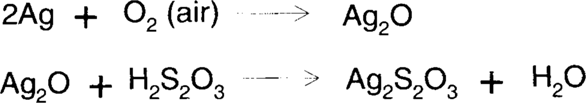
This treatment uses an acidified, rapid (ammonium) thiosulfate fixing bath to dissolve silver. The process depends on the removal of silver ions from solution by the formation of stable thiosulfate complexes to drive the process of air oxidation forward (Le Chatelier's Principle). The stability of a complex is given by its dissociation constant.

James also felt that Ag2O3 is an important silver oxide species in this type of reaction.3 but Ag2O3 very easily is converted to Ag2O.

The high acidity of the solution causes the formation of thiosulfuric acid (H2S2O3) from ammonium thiosulfate. The resulting silver thiosulfate (Ag2S2O3) is soluble in excess thiosulfate by the formation of higher thiosulfate complexes (most notably [Ag(S2O3)2]3− and [Ag(S2O3)3]5−).4
Both image silver and colloidal silver will be attacked, but the large surface-to-volume ratio of colloidal silver (such as is found in silver mirroring) makes the attack on colloidal silver much more rapid.
Like the ammonium-hypo reducer, ammonia/water treatment depends on the formation of stable complexes of silver. The Merck Index describes silver (I) oxide as “freely soluble” in ammonia.5 This is demonstrated by Linke. Approximately 0.022 g of silver (I) oxide will dissolve in a liter of water at 25°C.6 Tom Edmondson recommended using a 1% (v/v) solution of concentrated ammonium hydroxide (28%–30%).7 The resulting solution contains roughly 0.1479 moles of ammonia per liter of solution. Randall and Halford6 found that 0.1479 molar ammonia solution could hold 0.03499 moles of Ag+ ion per liter of solution. This is equivalent to half as many moles of silver (I) oxide or 4.05 g per liter of silver (I) oxide at 25°C. Olmer6 and Whitney and Melcher6 found higher solubilities, but neither group tested aqueous ammonia solutions as dilute as 0.1479 molar. However, even with Randall and Halford's results, it can be seen that the addition of approximately 2.5 g of ammonia to a liter of water increases the solubility of silver (I) oxide by a factor of over 180 times.
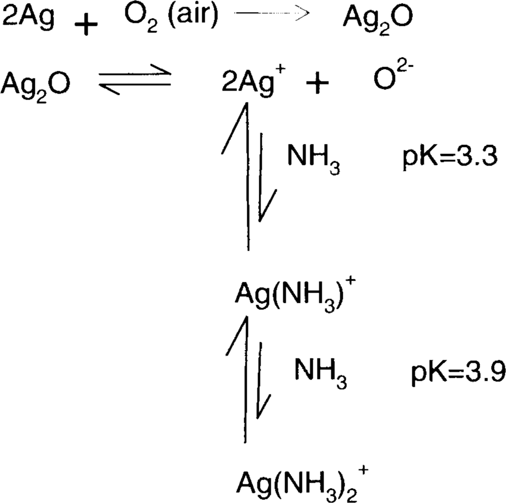
Removal of silver ion by complex formation drives the silver (I) oxide solubility towards dissolution (right), which in turn drives the air oxidation of silver towards the formation of more silver (I) oxide.
Gold chloride is technically a variation of bleach and redevelopment using gold chloride as the bleaching agent, but, in the process, gold metal is laid down. Gold has a higher reduction potential than silver, and therefore gold ions in the presence of silver metal will oxidize the silver to silver ions, while the gold is reduced to metallic gold. The presence of chloride ions without any chelating agents causes the silver ions to immediately precipitate as silver chloride, which can be redeveloped back into metallic silver. Therefore, the silver image is retained and is strengthened by the addition of gold. The problem is that, without at least a certain amount of chelating agent, the gold will deposit as either a yellow gold thiocyanate stain or as magenta (colloidal gold) stain. The trick then is to delicately balance the amount of thiocyanate; there should be enough to prevent the gold from coming out of solution, but not so much that the silver chloride is dissolved into solution as occurs in a toning process such as Kodak Gold Protective Solution GP-1. The problem of silver chloride formation in the photograph when this solution is used as a toner later in the process is solved by the use of a fixing bath after toning. This process was designed as an alternative to bleach and redevelopment for use with POPs. Bleach and redevelopment does not work with POPs.
In summary, the process is as follows:
The borohydrides are reducing agents that vary in reactivity to some degree with the attached cation. Sodium tends to be more reactive than potassium borohydride, but it's also hygroscopic.
The half-reactions (written as oxidations) are:
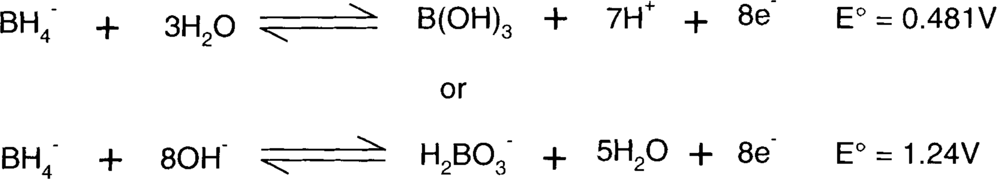
H2BO3− does not really exist, since boric acid (H3BO3 aka B(OH)3) is thought to be a Lewis acid and therefore an acceptor of OH-.8 The second half-reaction is more likely to be:
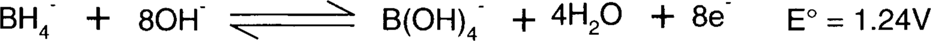
Borohydride, particularly sodium borohydride, will react with water to produce hydrogen gas.

The fact that hydrogen gas can also be used as an even milder reducing agent allows borohydride to be used with photographs directly in aqueous solution or indirectly in a chamber, such that the hydrogen gas evolved from aqueous solution performs the reduction.
The common use of reprocessing to restore deteriorated images appears to originate in the mistaken belief that poor processing (originally) is the cause of all deterioration. However, there are some grounds for its use. Primarily, the reduction of colloidal silver staining by reprocessing is a result of the oxidation and complexing of silver by oxygen and thiosulfate during fixing. In addition, some mild reduction bleaching may occur as a result of both the developer and the fixing bath.
Sodium bisulfite reduction of colloidal silver stain is similar in nature to ammonia and thiosulfate, in that it is dependent on the formation of stable silver ion complexes. Sodium sulfite, bisulfite, sulfurous acid, and sulfur dioxide form a series of equilibria. Bisulfite is a slightly more acidic sulfite salt than sodium sulfite. The actual pH of a mixed aqueous solution of sodium sulfite and bisulfite will depend on the relative concentrations of each species. Straight sodium sulfite is alkaline in water, while sodium bisulfite is acidic. Sulfite and bisulfite solutions gradually oxidize in air to form sulfate.
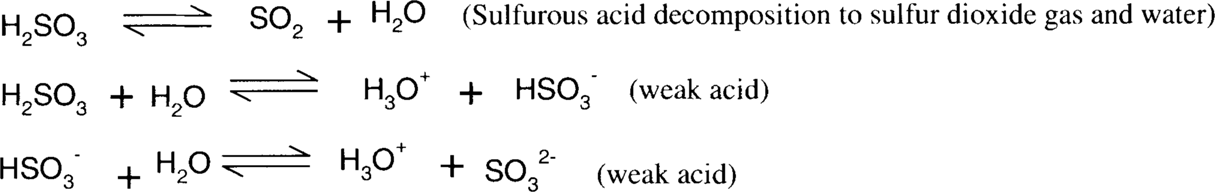
Similarly,
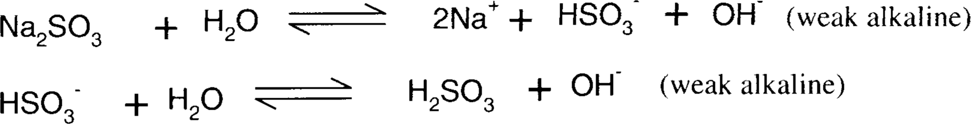
The complexes formed are much weaker than thiosulfate complexes and even ammonia complexes; therefore, this is much less effective than either ammonia or thiosulfate.
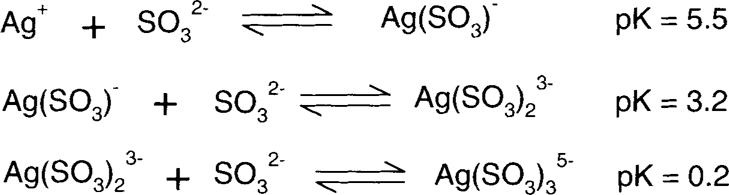
The low pK values show that the silver sulfite complexes are not very strong, so sulfite and bisulfite are not particularly good bleaches for colloidal silver.
For photographers, Farmer's Reducer is a mixture of potassium ferricyanide and sodium thiosulfate. The ferricyanide oxidizes silver metal to silver ion, which is then solubilized by the thiosulfate.
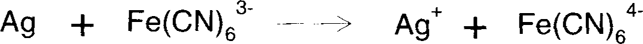
The ferricyanide solution can be used at various dilutions, allowing slower action and greater control. For conservators, the solution can be used in two parts. This method has several advantages. The mixed solution tends to lose potency rapidly because of the oxidation of thiosulfate by ferricyanide, which depletes both chemical components. Therefore, the separation of bleach from complexing agent allows the solutions to be used longer. Secondly, the over-bleaching can be reversed using a reducing agent such as developer or borohydride, as long as the silver hasn't migrated or been removed by thiosulfate. In this case, it would be advisable to add a precipitating agent such as sodium chloride to the bleach. This will immobilize the silver ion as silver chloride wherever it is formed.
Iodine oxidizes silver to form silver ion, which is immediately precipitated (immobilized) as silver iodide. The silver iodide can then be fixed out using a conventional fixing solution. Iodine is soluble in ethanol, and the dry alcohol is very slow to penetrate gelatin. The reaction is therefore initially limited to the surface, where silver mirroring is found, or to silver near the surface, such as redox blemishes.
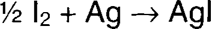
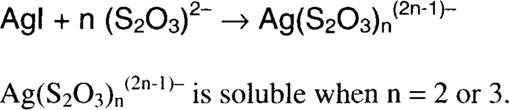
The dryness of the alcohol is very important to treatment control. The more water contained in the solution, the faster the iodine will attack the image silver.
Dissolve 1 to 10 g of analytical (reagent) grade iodine crystals in 1000 ml of dry ethanol. It is recommended that one of the formulations using methanol or methanol and 2-propanol, such as special formula 3a, be used rather than a formulation denatured with aviation fuel or butanone. Since 215 g of iodine should dissolve in 1000 ml of alcohol, 10 g should dissolve fairly easily. However, it can be very slow, and it is extremely important that all crystals have dissolved. Wyde originally recommended 0.1% iodine solution, but her later published formulation was a 1.0% solution. The stronger solution acts faster but is more difficult to control.
Treat the image fully or locally in the iodine solution. The mirroring will often take about three minutes to react, but the print must be watched carefully. Dry alcohol will eventually penetrate gelatin and will affect the image. The iodine solution is very opaque, and control can be a problem. This also makes it very difficult to determine whether or not all of the iodine crystals have dissolved. Treatment in a shallow bath of solution may make it easier to follow the progress of treatment but will also increase the rate of water absorption from the air.
Fix and wash as usual.
Paper Support Brightened
Iodine is a relatively mild oxidizing bleach.
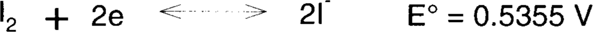
Compare with hydrogen peroxide:

Not all stains will be bleached by iodine but perhaps enough that the paper support generally looks brighter.
Loss of Yellowing in Highlights
Yellow colloidal silver has a large surface-to-volume ratio and therefore a large surface for attack for the amount of silver. Other factors may also be significant, but it is probably sufficient to refer only to the large surface-to-volume ratio. This causes the attack to be much faster on the colloidal silver particles than on filamentary silver. Silver filaments are roughly 100 times larger in diameter than yellow colloidal silver. Assume that both particles are roughly spherical in shape. The volume is therefore 4/3πr3 while the surface area is 4πr2, so the surface-to-volume ratio is 3/r or 6/d. The ratio of the surface-to-volume ratios of two particles is proportional to the ratio of their respective diameters. Therefore, the yellow colloidal silver particle has a surface-to-volume ratio approximately 100 times larger than a filamentary silver particle.
No Iodine Staining of Paper Support
Iodine is easily reduced by thiosulfate or sulfite to colorless iodide. Sulfite is typically found in fixing baths, particularly in acid-hardening fixing baths, as a preservative.
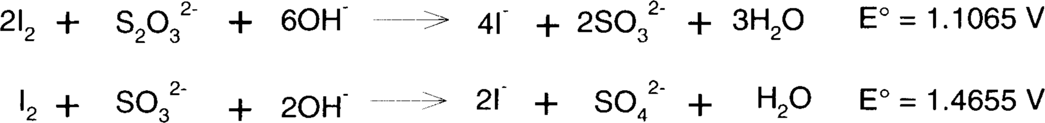
Slight acidity makes both thiosulfate and sulfite less favorable as reducing agents, but reduction of iodine will still occur. Very strongly acidic fixing baths will lose thiosulfate and sulfite as sulfur dioxide, so neither significant fixing nor reduction of iodine is likely.
Frilling and Gelatin Damage
This damage was particularly observed in glass plates, but not as much with DOPs. Photo conservators will recognize that this sounds typical of photographs stored under very dry conditions. As the gelatin binder dries out, it shrinks until the adhesion between the binder and support gives out. In paper prints, both the paper support and the gelatin emulsion will shrink as they dry out in air. In alcohol, gelatin will shrink, but the paper support will rapidly absorb alcohol and therefore does not. The gelatin will tend to shrink to a greater degree than paper, and this difference can be accommodated to some degree by curling of the print such that the gelatin is on the inside of the curl. Glass plates are dimensionally stable and won't shrink as the air dries out, while the gelatin will. There is therefore a greater difference in dimension between gelatin on paper and gelatin on glass, and, since glass doesn't readily curl, the gelatin adhesion to the glass gives out. (In theory, failure may occur in the gelatin, glass, or adhesion between the gelatin and glass.) The question then is why gelatin (and paper) may dry out in alcohol.
We know that gelatin contains some amount of water in equilibrium with the water vapor in the air. Dry alcohol is also notorious for absorbing water very rapidly out of the air. In fact, in analytical labs where very dry alcohol is required, the alcohol is often dried and then stored over a drying agent or molecular sieves in order to keep it dry. In either alcohol or gelatin, the water is considered to be in a condensed phase, although not necessarily liquid, such as in gelatin. If gelatin (and paper) is put into alcohol, thermodynamics requires that the water partitioned between the gelatin phase and alcohol phase must be in equilibrium such that the Gibbs free energy of water in each phase is equal. Technically, this is a three-phase system, since equilibrium must also be established with the air. However, assuming that the equilibration rate of the gelatin-alcohol system is significantly faster, we can ignore the air. Theoretically, the alcohol is dry; therefore, the only source of water is the gelatin, so there is a net movement of water out of the gelatin into the alcohol even if the alcohol doesn't actually penetrate the gelatin. Similarly, there is also a net movement of water out of the paper base in DOPs and POPs into the alcohol. Hence, the gelatin dries out and can suffer from physical damage particularly on glass plates.
Artificially Mirrored Samples Did Not Respond to Iodine-Alcohol Treatment
Artificially mirrored samples were created by oxidizing photographic silver using hydrogen peroxide, while hydrogen sulfide was used to nucleate the mirroring. This method works well to produce mirroring very rapidly, but unfortunately it creates mirroring with an unnaturally high silver sulfide content. It is also quite likely that much of the surface of the mirroring layer has been converted into silver sulfide. The silver in silver sulfide is already in the +1 state and can't be oxidized any further by the iodine. In addition, the solubility of silver sulfide is much, much less than that of silver iodide, so silver sulfide will preferentially precipitate over silver iodide. For comparison, over 230 million times as much silver will dissolve in water as silver iodide than as silver sulfide.
Iodine-Alcohol-Treated Negatives Increase in Stability over Time
Jesper Johnsen found that negatives treated in Weyde's iodine-alcohol solution appeared to increase in stability over time.9 This is not a great surprise. Henn et al. discussed the effect of iodide on the formation of redox blemishes on microfilm back in 1965. This led to the now standard practice of formulating microfilm fixing baths with the inclusion of potassium iodide.10 Steve Brandt studied the reactions of hydrogen peroxide on clean silver surfaces and on silver with anions adsorbed to the surface. He found that the decomposition of hydrogen peroxide into water and oxygen is catalyzed by a clean silver surface.11 One might ask, if a catalyst accelerates a reaction without being consumed by the reaction, how can the silver deteriorate? This has to do with the actual process of the decomposition reactions. Brandt demonstrated using a Pourbaix diagram that silver can cycle between the two oxidation states (Ag+ [silver ion] and Ag0 [silver metal]) using hydrogen peroxide as either a reducing or oxidizing agent to supply or consume electrons. The result is two reactions between silver and hydrogen peroxide.11
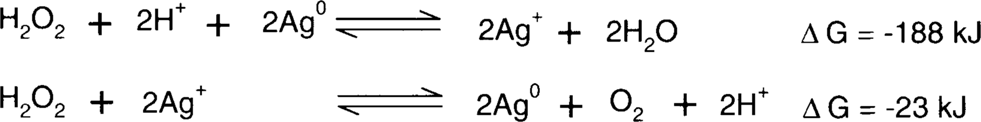
Net reaction:
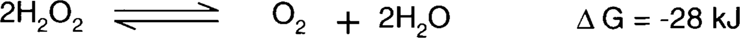
Note that the silver ion generated in one reaction is consumed by the second reaction leaving metallic silver again. The problem is that the silver ion migrates between the time that it was formed and when it will be consumed, forming orange-yellow colloidal silver. The presence of anions such as iodide or sulfide adsorbed to the surface of the silver prevents the silver surface from being used as a catalyst for the decomposition of peroxide. In effect, the iodide and sulfide behave like the vertical spikes on the roof of the Rochester City Hall that prevent pigeons from landing. Brandt said, “In the presence of a strongly specifically adsorbed species such as iodide, the catalytic surface of the silver is effectively poisoned, even at potentials at which phase AgI should not be present; therefore, the amount of energy required at the electrode surface to break the O-O bond is significantly increased.”12
Thermodynamically, silver in the presence of iodide or sulfide is much easier to oxidize.
Brandt showed that this effect was significantly outweighed by the slower peroxide reduction reaction.11 (Hydrogen peroxide acts as the oxidizing agent.)

Therefore, the iodide generated during treatment with Weyde's iodine-alcohol solution can adsorb to the surface of the silver to improve the resistance to oxidation by peroxides. Further enhancement will result from the adsorption of thiosulfate ion during the fixing step. Be aware that excessive washing will remove the adsorbed anions and negate the possibility of improved stability.
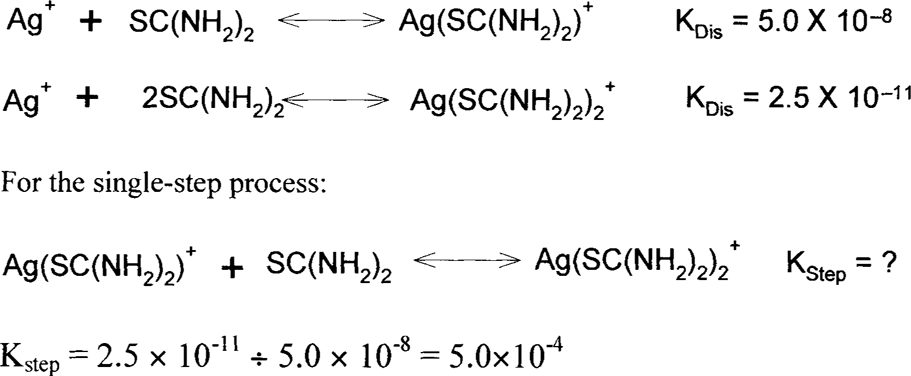
Acid-thiourea formulas depend on the same theory as ammonium thiosulfate, ammonia, and bisulfite. Metallic silver, particularly in colloidal particles, is oxidized by air. Silver (I) oxide in the presence of acids forms silver ions, and these silver ions are held in solution as one or several silver complexes.
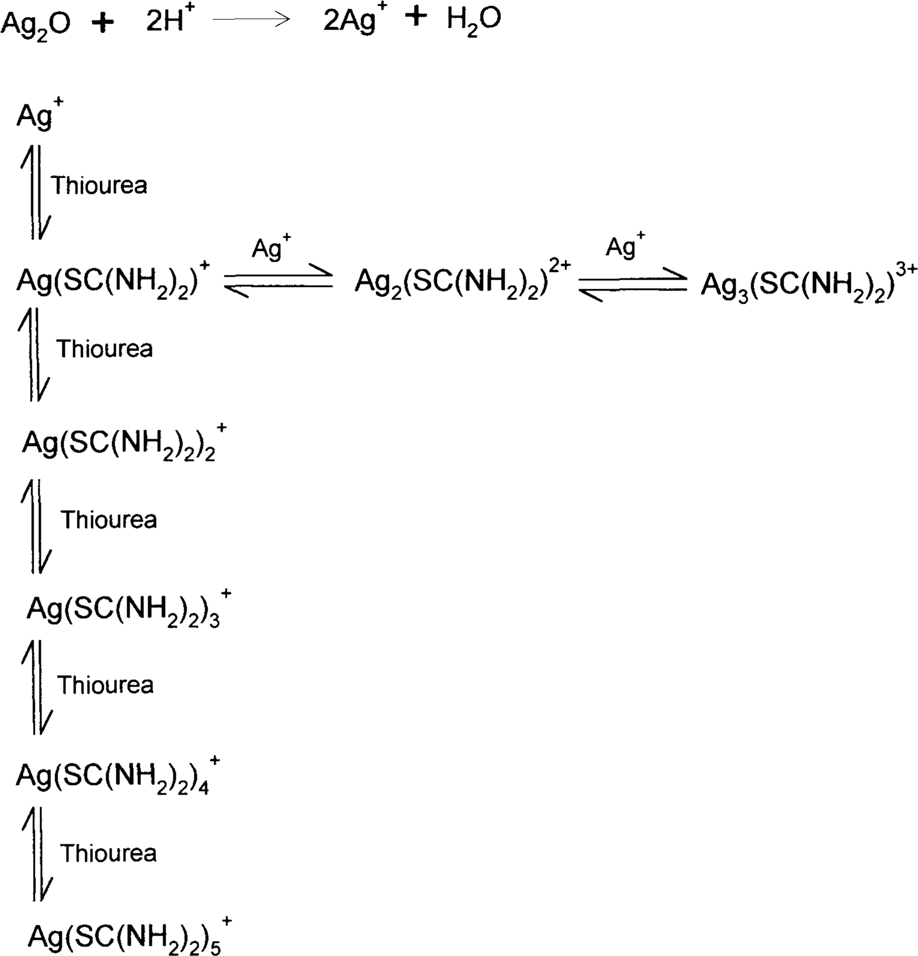
These are the single-step dissociation constants for the reactions above.
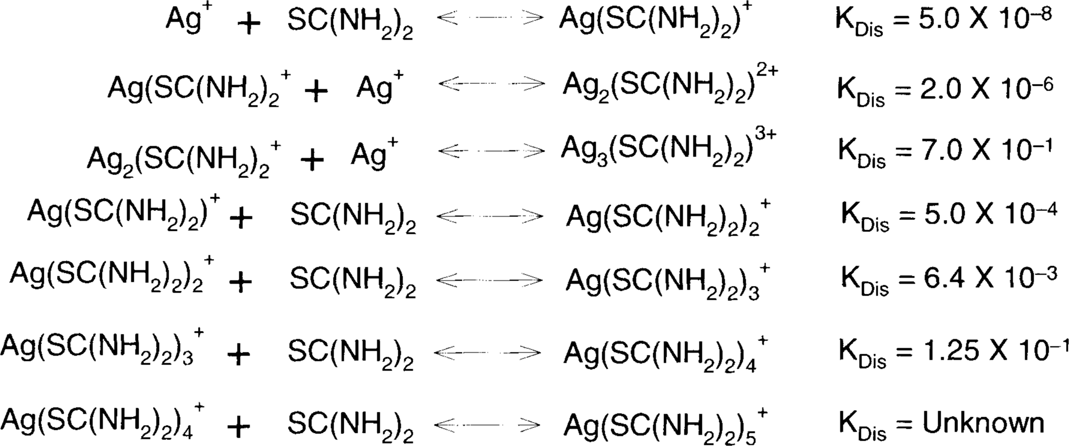
Solubility data and observations suggest that only the complexes with higher ratios of thiourea to silver are soluble. In fact, thiourea shows behavior similar to that of thiosulfate in this regard. For example, 23.7 g of AgCl.3(SC(NH2)2) will dissolve in a liter of water while 786.34 g of AgCl.5(SC(NH2)2) will dissolve in a liter of water.13 Na(AgS2O3).H2O is described as “sparingly soluble” and Na3[Ag(S2O3)2].2H2O is “readily soluble.”14 T. H. James commented, “When thiourea is added to a silver nitrate solution the precipitate that is first formed dissolves when the mole ratio of thiourea to silver is approximately 3:1.”15 Since silver nitrate is extremely soluble in water, the initial precipitate must be silver thiourea complexes with a high silver-to-thiourea ratio. As the composition of the complexes becomes higher in thiourea, they become soluble. These soluble complexes will be promoted by a high concentration of thiourea in the solution.
It was found during the workshop that more dilute solutions acted more slowly and were therefore easier to control. The question is: how dilute can one go? The complex dissociation constant shows that, for a fixed concentration of silver, the lower the concentration of thiourea, the lower the concentration of the complex. In other words, as the thiourea concentration is reduced, the solution will hold less silver, just as lower concentrations of thiosulfate in a fixing bath will cause the fixing bath to reach exhaustion sooner (with less silver in solution.) It was found that the solubilities for silver bromide and silver iodide in thiourea support this hypothesis. The C. Fischer formula should contain silver citrate, which is also very slightly soluble in water, but thiourea data could not be found for silver citrate. Data have been obtained from various sources, and some were recalculated to more or less put everything into the same terms.
| Silver salt | Temperature | Thiourea conc. | Soluble g of silver salt per 100 g water |
| AgBr16 | 25°C | 9.09% (w/w) | 1.87 |
| AgBr16 | Room | 1% | 0.149 |
| AgI17 | 25°C | 9.09% (w/w) | 0.79 |
| AgI17 | 25°C | 0.99% (w/w) | 0.008 |
Very low concentrations of thiourea may cause the formation of insoluble complexes. Silver citrate formed by the reaction of citric acid with silver (I) oxide is very slightly soluble: 0.0277 g at 18°C and 0.0284 g at 25°C per 100 g water.18 Using James as a guide, assume that a minimum of 3:1 (mol:mol) ratio of thiourea to silver is required. The C. Fischer formula as mixed should dissolve less than 1.9 g of silver in 200 ml at saturation. Note that James used silver nitrate, which is very soluble. Roughly 69 g of silver nitrate will dissolve to make 100 g of saturated solution. This means 69 g of silver nitrate dissolves in 31 g of water. The proposed silver limit for the ISO standard for black-and-white print stability is 0.025 g/m2 or 0.0013 g in an 8 × 10-inch print.19 This means that if the C. Fischer solution is saturated with silver, an 8 × 10-inch print must absorb less than 0.14 ml of solution. Exhaustion in typical sodium thiosulfate-based fixing baths is considered to occur when the molar ratio of silver to thiosulfate is 1:52 for prints and 1:17 for film, even though the soluble complexes form with a ratio of roughly 1:2 or 1:3. Obviously, real research needs to be done on this topic, but until then conservators are advised to dilute with caution and to replace the solution frequently if large prints or large numbers of prints are to be treated. Dilution to half-strength is probably safe, since the resulting solution is similar to the following one recommended by J. Hauff in 1894 for the safe removal of dichroic fog.20
| thiourea | 1 g |
| citric acid | 1 g |
| water to make | 100 ml |
To 200 ml distilled water, dissolve 4 g of thiourea (ACS reagent grade), and 2 g of anhydrous citric acid (ACS reagent grade).
Note that the National Toxicology Program Cancer Classification for thiourea says, “Reasonably anticipated to be a carcinogen.” Appropriate clothing and safety equipment as well as extreme care must be used when handling this chemical. (See Appendix B.)
Presoak in water for one minute.
Treat fully or locally in acid-thiourea solution. Remove solution (or rinse local area with water) immediately at the first sign of image bleaching.
Wash 15 minutes.
Highlights and Mid-Tones May Be Bleached
The bleaching reaction is faster with yellow colloidal silver, but the bleach will still react with the image. The mid-tones and highlights have less silver available, so the loss of image will be visible in these areas first.
Little Image Silver Remained in Stained Areas after Treatment
The yellow colloidal silver stain is formed from silver that migrated from image particles. Therefore, the greater the amount of yellow stain, the less image silver there is left.
Slight Changes in Surface Character
Thiourea, like thiocyanate, causes gelatin to soften. Many experiments have been performed in the photographic industry to see if larger organic derivatives of thiourea, such as tetramethylthiourea and ethylenethiourea, could successfully be substituted for thiourea for stabilization processing without the softening problem, but without much success.
Care must be taken during treatment, especially during local application, not to damage the softer gelatin. Prehardening with a formaldehyde hardener may help, but the absorption of the acid thiourea solution will potentially be slower.
Works with Colloidal Silver Stain, but Not Sulfiding
The sulfide half of silver sulfide can't be oxidized by air the way metallic silver can; therefore, silver sulfide can't react quite as easily as silver. However, acid-thiourea formulas are successfully used to remove silver tarnish products including silver sulfide on silver objects such as silverware. The dissociation of silver sulfide can be driven forward by both the consumption of the freed silver ion by complexing agents such as thiourea and by the removal of the sulfide in acid solution as hydrogen sulfide gas. In theory, it should be possible to remove silver sulfide by acid-thiourea, but the gelatin binder may interfere.
Not Very Successful with POP
Some people at the workshop were successfully able to treat POPs, although problems are possible. POP images are produced from essentially colloidal silver particles that will also be very susceptible to attack. Mirroring may be easier to remove than colloidal silver stain on POPs simply because mirroring is on the surface and will react slightly faster than silver further in the binder layer.
Stained Areas Less Planar After Treatment
This is one observation that is difficult to explain. Both thiourea and acidity are consumed in stained areas, but there is no obvious reason why lower concentrations at the immediate surface of either of these substances should cause a different dimensional character.
Thiourea contains at least one sulfur atom that has an oxidation number of −2 which makes it an active sulfur compound. It will adsorb to the silver surface and may form silver sulfide.
James and Vanselow21 showed that, in alkaline solution, thiourea adsorbed to silver ions would decompose to form silver sulfide and cyanamide.
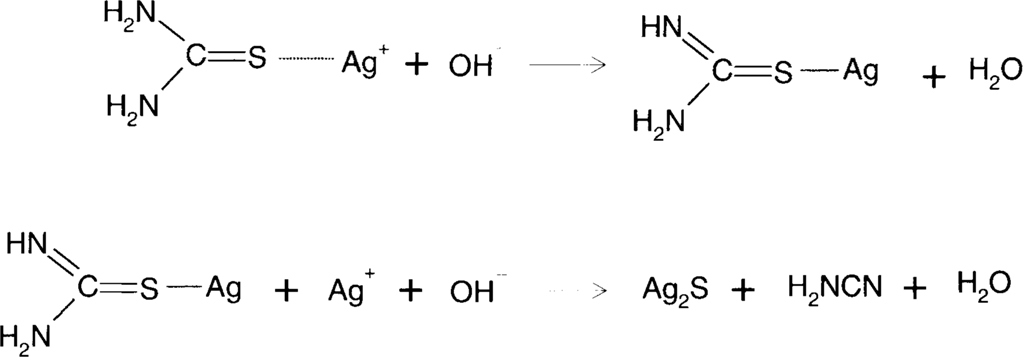
There are two obvious solutions to reducing this problem. One is to make sure that the acidity level is maintained. The reaction of silver oxide with acid consumes the acid and makes the solution more alkaline. The second thing to do is to make sure that there is adequate thiourea present. Higher levels of thiourea lower the concentration of Ag[SC(NH2)2]+ which is the starting species for the above decomposition reaction. Complexes with higher ratios of thiourea to silver will preferentially form. Alternatively, one could keep the silver concentration in solution low by not overusing the solution. Optimally, fresh solution should be used for each treatment.
To prevent silver sulfide formation in the future, it is important to remove both thiourea and the silver thiourea complexes after treatment. This operation is very much like conventional fixing and washing. The ratio of silver to thiourea must be low enough in the treatment solution that the soluble higher thiourea complexes are formed. These should be removed in the treatment solution. A concentration equilibrium will form between the silver thiourea complexes in the print and in solution. Since the stepwise dissociation constants for the higher complexes are not very low, these complexes will tend to break down towards higher silver-to-thiourea complexes which are less soluble. Here, then, is another reason for caution when using diluted versions of the C. Fischer formula. Thiourea, like thiosulfate, should wash out of the emulsion relatively easily.
It is probably not necessary to go to great lengths to try to remove the thiourea from the photograph. There is evidence that, like thiosulfate, a small amount of residual thiourea can be beneficial to the stability of the image silver. Henn and Mack, for example, in optimizing the formulation of Gold Protective Treatment GP-2 noted, “Further improvement in stability of the image is obtained if the thiourea content is increased to 4x or 10x the gold content, and this has been done in Formula 2.”22 Experiments at IPI demonstrated that GP-2 had a much greater image stabilizing effect than a similar solution using a complexing agent with a non-active sulfur (thiocyanate.)23 Even acidified thiourea can provide some improvement to image stability. IPI found that GP-2 without gold (essentially an acidified thiourea solution) and plain sodium sulfide solution both inhibited discoloration in the high-density areas during peroxide fuming.24
The remaining question is whether thiourea can be washed out of the paper base easily or whether it is strongly adsorbed to the paper fibers like thiosulfate.
Paul was apparently also curious about how well thiourea washed out of paper, so he ran an experiment. He treated what he described as “conservation grade” blotter paper with the C. Fischer formula and then washed in several ways. If the thiourea behaves like thiosulfate, then depending on the treatment time, the thiourea should adsorb to the cellulose and be difficult to remove by washing.
His five samples were:
The samples were analyzed for sulfur by Richard Newman, Head of Scientific Research, Museum of Fine Arts, Boston, using wavelength-dispersive X-ray fluorescence (WDS) in an electron beam microprobe.
All of the treated and washed samples contained small amounts of sulfur, but all less than the control. The unwashed sample contained about 50 to 100 times the sulfur level of the washed samples.
Paul's results indicate that thiourea can be easily washed out of paper and should not be a cause for concern.
For more information, see Appendix C. It should be noted that the text is an informal email message from Paul and not a formal letter or report. The message is reproduced with the permission of Paul Messier and Richard Newman.
Le Chatelier's principle says that if a system is at equilibrium, any change (stress) imposed on the system will result in the equilibrium shifting such that the change is nullified. The obvious thing to do to drive a reaction forward might be to increase the concentration of reactants or decrease the concentration of product. Products that precipitate out of solution are effectively removed from the reaction going on in solution, and this can push the reaction forward. Other possible stresses are changes in temperature, pressure, or volume. The Haber process for making ammonia directly by the addition of hydrogen gas and nitrogen gas is an excellent example.
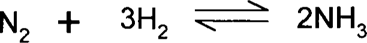
There is a larger number of gas molecules (and larger volume) associated with the reactant side than the product (ammonia) side; therefore, increasing the pressure will tend to drive the reaction forward so that the pressure will decrease. The reaction generates heat, so it will tend to be driven forward if the reaction chamber can be kept cool, although this must be balanced with the problem that the reaction rate slows down too much if the reaction chamber gets too cold. Industry utilizes Le Chatelier's principle by optimizing the temperature between too hot (driven towards the reactant side) and too cold (too slow); by running at high pressure with an excess of nitrogen or hydrogen gas; and by constantly removing ammonia gas. So, four things tend to drive the reaction forward: temperature, pressure, reactant concentration, and product concentration.
This constant is really a measure of how strongly the complex is held together. Chemists look at the general silver-complex equilibrium as:
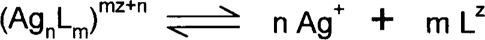
If we have silver ions in solution with three times as many thiosulfates, the equation is:
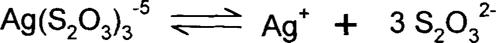
Note that the charge on the three thiosulfates is −2 and the charge on the single silver ion is +1, so the charge on the complex is (3 × (-2)) + (1 × (+1)) = −5.
The dissociation constant K is given by:
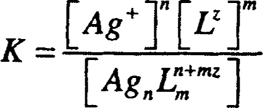
where each of the square brackets represents the activity (more or less the concentration) of each of the three species. The smaller the K value is, the stronger the complex. Therefore, at equilibrium there will be a great tendency to form the complex. Some chemists use the formation or stability constant, which is the reciprocal of the dissociation constant (1/dissociation constant). Many complex formations occur in steps, and the step-wise dissociation constants can be determined by division.
Often, in order to save having to write scientific notation, KDis and KStep values are often written as the negative base 10 logarithm. (Take the log of the value, not the natural log, In, and then switch the sign. If it is negative, then it becomes positive and vice versa.) This is the same thing that we do with H+ concentration to get pH, and we use a similar notation, pKDis or pKStep. So a K value of 5.0 × 10−8 has a pK value of 7.3.
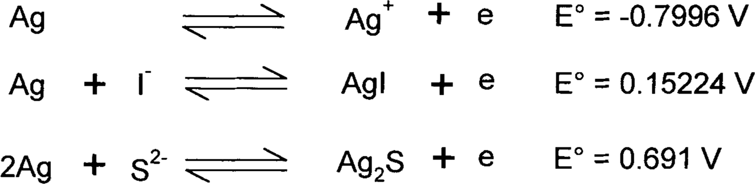
These are oxidation-reduction reactions, usually abbreviated as “redox.” In these reactions, one reactant loses electrons (oxidation) while another gains electrons (reduction). Virtually any compound can act as a reducing agent (a species that loses electrons to another) or an oxidizing agent (a species that pulls off electrons from another). How a compound will behave depends on its strength. It can be strong enough to pull off electrons from another reactant or it can lose electrons to a stronger reactant in the reaction. In order to make things easier, compounds are tested under standard conditions versus a standard hydrogen electrode (although you may find other reference standards used in some literature). The reaction of a single compound possibly including H+ (acid), OH− (base), and/or H2O is called a half-reaction and is written as a reduction reaction by convention. (Older literature may write it as an oxidation reaction.) In other words, the reactions are written such that the compound gains electrons going from the left side of the reaction (reactant) to the right side (product). Ag+ + e → Ag is an example of a reduction half-reaction in which the silver ion (Ag+) gains an electron (e) to form silver metal (Ag). The affinity that the Ag+ has for the electron is given as an E° value in volts versus the standard hydrogen electrode. E° for the standard hydrogen electrode is then given the value of zero. Be aware that E° for the various compounds is measured under specific standard conditions. The actual value, E, will depend on several variables including concentration of the various species. Corrections to E° can be calculated using the Nernst equation, but that is beyond the scope of this paper. For a reaction to be spontaneous, the Gibbs free energy must decrease (be less than 0). If the free energy is zero, the reaction is at equilibrium, and the net result is that nothing measurable is happening.
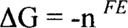
where n is the number of electrons involved in the reaction and F is Faraday's constant. What is important is E. ΔG is negative (spontaneous) only when E is positive. (Most textbooks use the script E, but we're calling it E.
To get oxidation reactions, the reduction reaction is mirrored left to right so that the right side is now on the left and the left side is on the right. For example, Ag → Ag+ + e is now in the form of an oxidation reaction. The E° value is also switched, so that positive values become negative and vice versa. Now, how do these half-reactions combine?
Here is an example from above: the reduction of water to hydrogen by borohydride. The two half-reactions (as reductions) are:
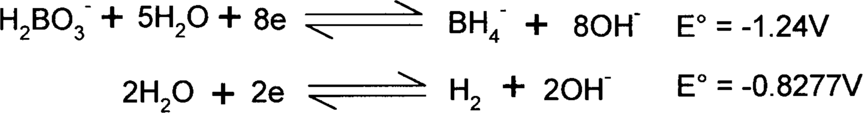
The borohydride (BH4−) is supplying the electrons, so it must be on the left side. The same number of electrons must be supplied and consumed, so the second reaction will be multiplied by 4. This will not change the E° value, though.
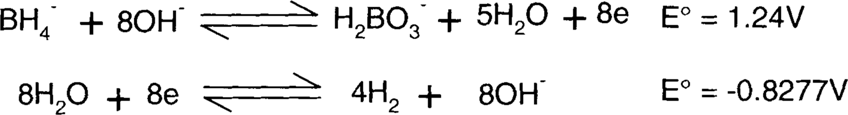
Each side of the reaction is added together.
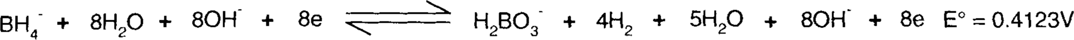
Finally, species found on both sides of the reaction, such as water, hydroxide ions, and electrons, are subtracted.
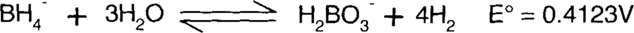
Since the E° value is positive, this is a spontaneous reaction. Note that both the left and right sides of the equation balance in both atoms and charge. Both sides have a single negative charge, one boron atom, ten hydrogen atoms, and three oxygen atoms.
Enthalpy is a measure of the total available thermal energy in a system of atoms or molecules. It is the sum of the kinetic and potential energy of the particles as well as the pressure and volume. It is difficult to imagine that pressure multiplied by the volume could be an energy measurement. When one pumps up a bicycle tire, the pump gets hot. Some of this heat is due to friction, but not all of it. When the pressure in the tire is released, the air and tire cool down, although it is difficult to feel this cooling effect because of the elasticity of the inner tube and total volume of air. However, if a high-pressure tank of air is opened (in a controlled way), the tank will cool down and may even form frost. A CO2 fire extinguisher is another example of the energy contained by pressure and volume. The high-pressure, room-temperature compressed gas in the fire extinguisher becomes a snow of dry ice when the pressure is released.
Kinetic energy is easy to understand, but where might potential energy come from? In high school physics we learn that raising the height of an object increases the potential energy, but this is gravitational, not thermal, potential energy. We see potential energy contained in humid air. This potential energy is important to HVAC engineers. The energy required for environmental control is a function of the required enthalpy change in the air, among other things. Warmer air contains greater enthalpy, which makes obvious sense. However, enthalpy also goes up as humidity increases at any constant temperature. This is because it takes energy to put the moisture into the air. This is also why sweating keeps us cool. Engineers refer to the humidity as “latent heat,” since we can't feel it by touch, but it is there, nonetheless. If I start with a gram of water on my hand at body temperature, it will take just over 500 calories to convert that water at 37°C to water vapor at 37°C. That extra heat energy is needed to make the phase change. Similarly, approximately 80 calories is needed to change 0°C ice to 0°C water. (In the chemistry literature, “food” calories are spelled with a capital “C” and are really kilocalories. In popular use, both types of calories as spelled with a lower-case “c”.)
We've all heard that spontaneous reactions occur only if the disorder or entropy increases. This is why it's so easy to convert gasoline into carbon dioxide and water (with a few other odds and ends), but it's very difficult to take carbon dioxide and water and convert it into gasoline. All it takes is a match to make the reaction go in the spontaneous direction. However, the idea of increasing entropy is only true in a thermally isolated system. Joshua Willard Gibbs determined that the amount of useable chemical energy available in a reaction must always decrease in a spontaneous reaction at constant temperature and pressure. This quantity of “free” energy is called Gibbs free energy. It can be calculated for systems with changing temperature and pressure, but the interpretation is much more complex. One form of his equation shows that it's possible for a spontaneous reaction to occur with decreasing disorder as long as enough heat energy is lost. This sounds very complicated. Consider a situation in which we have supercooled water at −1°C. If very pure water is cooled undisturbed it can be cooled fairly far below the freezing point without turning to ice. However, a slight disturbance will spontaneously change the water to ice. In other words, a disordered system is spontaneously changing to a more ordered one (in defiance of our ideas about increasing entropy). In the process of freezing, the temperature remains the same, but the ice must lose about 80 calories per gram. This loss of heat more than compensates for the decrease in entropy.
Gibbs free energy is important when we look at multi-phase systems such as water in gelatin and water in alcohol. It's also important in determining whether or not an oxidation or reduction reaction will occur. (See Electrochemical Reactions.)
The Lewis theory of acids and bases is the most general of the three common theories. To understand the Lewis theory we should start with the simplest and most restrictive definitions. Arrhenius defined an acid as a substance that produces a hydronium ion in water (H3O+). For example, hydrochloric acid (HCl) in water produces H3O+ + Cl−. A base is defined as producing OH− in water. Neutralization occurs by the combination of the hydronium ion with the hydroxide to produce two water molecules. So far, so good. What happens if we start with HCl gas and NH3 (ammonia) gas? The two gases mix together and produce a white powder, NH4Cl, ammonium chloride, a neutral salt. No water, hydronium ions, or hydroxide ions are involved. This led to the Brønstead or Brønstead-Lowry theory.
Brønstead and Lowry defined an acid as a proton or H+ donor (a hydrogen atom is a proton with one electron, so a positive hydrogen ion is simply a proton), while a base was defined as a proton acceptor. This theory covered not only aqueous solutions but also gases, solids, and non-aqueous solvents.
It was realized that the one thing all of the bases as defined by the Brønstead-Lowry theory had in common was that all of them had at least one pair of unshared valence electrons. (Valence electrons are those that are outermost and are responsible for chemical bonding.) Lewis then defined bases as electron pair donors, which meant that acids had to be electron pair acceptors.
This definition encompassed all of the substances defined as acids and bases by the Brønstead-Lowry theory as well as many other compounds. Boron trifluoride (BF3) has only three pairs of electrons and is effectively missing a pair to make a complete octet. (It has long been considered that atoms with eight valence electrons are most stable and that atoms will tend to bond in such a way as to make octets of electrons for each atom.) Boron trifluoride is therefore defined as a Lewis acid, and it will react with things like ammonia (which has a free pair of electrons available).
Boric acid is similar to boron trifluoride in that it has only three pairs of electrons. It is therefore thought to behave as an acid, not by losing one proton, but by bonding to the available pair of electrons on a hydroxyl ion. This makes boric acid, B(OH)3, become B(OH)4− in the presence of OH− rather than H2BO3−.
1. Stodulski, L. P., V. Baas, and D. Severson. “A Preliminary Investigation Into the Effects of Aqueous Sodium Borohydride Solutions on Silver-Based Photographic Materials.” Topics in Photographic Preservation, vol. 3, Compiled by Robin E. Siegel, attachment. Washington: American Institute for Conservation, Photographic Materials Group, 1989.
2. Baas, V., J. J. Bischoff, and L. Stodulski. “Ongoing Investigations into Chemical Image Enhancement of Faded Vintage Printing-out Photographic Prints.” Topics in Photographic Preservation, vol. 5, Compiled by Robin E. Siegel, 95–116. Washington: American Institute for Conservation, Photographic Materials Group, 1993.
3. James, T. H. The Theory of the Photographic Process, 4th ed. New York: MacMillan Publishing Co., Inc. 1977. p. 448.
4. Haist, G. Modern Photographic Processing, vol. 1. New York: John Wiley & Sons, Inc. 1979. p. 562.
5. Budavari, S., ed. The Merck Index, 11th edition. Rahway (NJ): Merck & Co., Inc. 1989. p. 1349.
6. Linke, W. F., Solubilities: Inorganic and Metal-Organic Compounds: A Compilation of Solubility Data from the Periodical Literature, Volume I: A – Ir. New York: D. Van Nostrand Company, Inc. 1958. pp 124–125. Copyright held by the American Chemical Society.
7. Edmondson, T. M. “Chemical Treatment of Photographs: Ammoniated Water Treatment for Silver Mirroring,” Chemical Treatment of Photographic Materials Workshop Handouts, compiled by Debbie Hess Norris and Nora Kennedy. 1999.
8. Cotton, F. A., and G. Wilkinson. Advanced Inorganic Chemistry: A Comprehensive Text, 3rd ed. New York: John Wiley & Sons. 1971. p. 230.
9. Johnsen, J. S. “The Use of Study Collections for Examination and Teaching in Chemical Treatment of Bleached and Discolored B&W Negatives.” Paper presented at the Interim Meeting of the ICOM-CC Working Groups Graphic Documents and Photographic Documents, Ludwigsburg, April 20–22, 1998.
10. Henn, R. W., D. G. Weist, and B. D. Mack. “Microscopic Spots in Processed Microfilm: The Effect of Iodide.” Photographic Science and Engineering 9 (1965): pp. 167–173.
11. Brandt, E. S.. “Mechanistic Studies of Image Stability. 3. Oxidation of Silver from the Vantage Point of Corrosion Theory.” Journal of Imaging Science 31 (1987): p. 201.
12. Brandt, E. S.. “Mechanistic Studies of Silver Image Stability. 1: Redox Chemistry of Oxygen and Hydrogen at Clean and at Adsorbate-Covered Silver Electrodes.” Journal of Imaging Science 28 (1984): p. 9.
13. Link, W. F. op. cit., p. 78.
14. James, T. H. op. cit., p. 439.
15. James, T. H. op cit., p. 446.
16. Link, W. F. op. cit., p. 17.
17. Link, W. F. op. cit., p. 99.
18. Link, W. F. op. cit., p. 46.
19. ISO, International Standard for Imaging Materials – Wet-Processed Silver Gelatin Type Black-and-White Reflection Print – Specifications for Non-Display Stability, Working Draft 2. Geneva: International Organization for Standardization (ISO). 2000. p. 16.
20. Clerc, L. P. Photography: Theory and Practice, 3rd ed., London: Sir Isaac Pitman & Sons., Ltd. 1954. Originally published as La Technique Photographique, p. 326.
21. James, T. H., and W. Vanselow. “Relative Rates of Reaction of Silver Bromide with Adsorbed Monolayers of Derivatives of Thiourea,” The Journal of Photographic Science: Section B of the Photographic Journal 1 (1953): p. 133.
22. Henn, R. W., and Mack, B. D. “A Gold Protective Treatment for Microfilm,” Photographic Science and Engineering 9 (1965): p. 383.
23. Gmuender, C. “On Black-And-White Paper Image Stability Enhancement Effectiveness of Toning Treatments on Silver Gelatin Prints Determined by the Hydrogen Peroxide Fuming Test.” Master's thesis, Rochester Institute of Technology, 1992. pp 46–47.
24. Reilly, J. M., D. W. Nishimura, K. M. Cupriks, and P. Z. Adelstein. “Polysulfide Treatment for Microfilm,” Journal of Imaging Technology 17 (1991): p. 102.
The following notes were provided by Mogens Koch from the School of Conservation at the Royal Academy of Fine Arts in Copenhagen. Koch noted that chemical treatments are used fairly commonly in Europe, while they're rarely applied in North America. Appendix B consist of translations, transcriptions, and notes by Mogens Koch, which are reproduced here with his permission. Be aware that the fractional measurements in formulas use the European comma rather than the North American decimal point. Agfa Agepon is similar to Kodak Photo-Flo 200.
During a visit to Rochester in December 2000, Koch mentioned that they had observed that the integrity of the gelatin during bleach and redevelopment was strongly affected by the composition of the wash water (tap water). Apparently identical chemistries reproducibly worked safely in some cities and not in others.
Five notes follow:
The method is based on the transformation of the silver mirror surface on the negative's emulsion into silver halide, after which it is removed with hypo. The method has been worked out by Dr. Edith Weyde.
Negative material on glass, plastic, or paper materials, which can withstand treatment in alcohol (ethanol C2H5OH) and water.
Materials with collodion emulsions may never be treated with iodine-alcohol, as the collodion emulsion is dissolved completely by alcohol solutions.
It holds for every type of material that its present state of preservation, its age, and contents should be decisive factors, which are taken into account before a treatment is decided upon.
The method can be used for oxidation of silver mirror, which is caused by the metallic silver, on oxidation, being transformed into colourless silver ions. The ions migrate through the emulsion to the surface where they are reduced to metallic silver.
Silver mirror shows itself as a metallic, glittering film over the whole or part of the image when seen in reflected light. In penetrating light, silver mirror shows itself as a pale brown area.
Silver mirror is usually formed due to a reaction between image silver and atmospheric air. The damage is intensified in case of polluted air especially sulphur. Similarly, negatives that were insufficiently treated in the developing process and/or have been kept in unsuitable packing will be more sensitive to the development of silver mirror. Finally, damp stains formed on photographic emulsions before exposure may cause silver stains on the emulsion.
6 photo trays, which are twice the size of the negatives to be treated
1–2 rinsing possibilities, where chemicals can be rinsed off the negatives
Stopwatch
Safety equipment
Iodine (I2)
Ethanol/alcohol pure (>96% C2H5OH)
Agfa Agepon
Ilford Hypam Rapid Hypo. Normal fixing bath for negatives
Kodak Hypo Clearing Agent
| Iodine | 1 g |
| Alcohol >96% | 1000 ml |
| Agepon | 1 part |
| Water | 200 parts |
Description of state of preservation, etc. is made before any sort of treatment is instigated.
The negative is cleaned mechanically. Dirt is brushed off.
A safety copy is made.
The negative that is to be treated is first placed in the iodine-alcohol solution for approx. 2–3 minutes, where it is constantly moved about.
Remember that the negative must be completely dry when placed in the iodine-alcohol solution, as the negative is only to be treated on the surface. The solution may not penetrate the emulsion and transform the image creating silver into silver iodide. Iodine is an oxidizing agent which can oxidize the metallic silver in the silver mirror into silver ions, as it itself is reduced to iodine ions.
To keep any iodine residue from affecting the image silver, the negative is placed in a bath of pure alcohol. Treatment time approx. 1 minute.
The ethanol forces a large amount of water out of the emulsion, which causes the emulsion to shrink. For this reason, the negative is treated in Agepon, which acts as a detergent. Treatment time approx. 1 minute.
In order to remove the dissolved stains and to stabilize the negative against future deterioration, it is now placed in a fixing bath for 10 minutes.
The negative is then rinsed in running water of not over 20°C for at least 1 minute.
In order to facilitate the rinsing out of remains of hypo, the negative is treated for approx. 3 minutes in 20°C Hypo Clearing Agent.
The treated negative receives a final rinse for at least 10 minutes at approx. 20°C or colder.
The negative is treated in an Agepon bath for 1 minute.
The negative is dried, away from heat and dust.
Source: E. Weyde. First time printed in “Handbuch der Negativ-Restaurierung”, Bildarchiv Foto Marburg 1977. Unofficial book.
The method is based on the transformation of the silver mirror surface on the positive's emulsion into silver halide after which it is removed with hypo. The method has been worked out by Dr. Edith Weyde.
Positive material on glass, plastic or paper materials which can withstand treatment in alcohol (ethanol C2H5OH) and water.
Materials with collodion emulsions, be they direct positives or positives, may never be treated with iodine-alcohol, as the collodion emulsion is dissolved completely by alcohol solutions.
It holds for all types of materials that its present state of preservation, its age and contents should be decisive factors, which are taken into account before a treatment is decided upon.
The method can be used for oxidation of silver mirror, which is caused by the metallic silver, on oxidation, being transformed into colourless silver ions. The ions migrate through the emulsion to the surface where they are reduced to metallic silver.
Silver mirror shows itself as a metallic, glittering film over the whole or part of the image when seen in reflected light. In penetrating light silver mirror shows itself as a pale brown area.
Silver mirror is usually formed due to a reaction between image silver and atmospheric air. The damage is intensified in case of polluted air - especially sulphur. Similarly, positives that were insufficiently treated in the developing process and/or have been kept in unsuitable packing will be more sensitive to the development of silver mirror. Finally, damp stains formed on photographic emulsions before exposure may cause silver stains on the emulsion.
6 photo trays which are twice the size of the positives to be treated.
1–2 rinsing possibilities, where chemicals can be rinsed off the positives.
Stopwatch.
Safety equipment.
Iodine (I2)
Ethanol/alcohol pure (>96% C2H5OH).
Agfa Agepon
Ilford Hypam Rapid Hypo. Normal fixing bath for positives.
Kodak Hypo Clearing Agent.
| Iodine | 1 g |
| Alcohol >96% | 1000 ml |
| Agepon | 1 part |
| Water | 200 parts |
Normal concentration for positives
Normal concentration
Description of state of preservation etc. is made before any sort of treatment is instigated.
The positive is cleaned mechanically, dirt is brushed off.
A safety copy is made.
The positive, which is to be treated, is first placed in the iodine-alcohol solution for approx. 2–3 minutes, where it is constantly moved about.
Remember that the positive must be completely dry when placed in the iodine-alcohol solution, as the positive is only to be treated on the surface. The solution may not penetrate the emulsion and transform the image creating silver into silver iodide. Iodine is an oxidizing agent which can oxidize the metallic silver in the silver mirror into silver ions, as it itself is reduced to iodine ions.
To avoid that any iodine residue affects the image silver, the positive is placed in a bath of pure alcohol. Treatment time approx. 1 minute.
The ethanol forces a large amount of water out of the emulsion, which causes the emulsion to shrink. For this reason the positive is treated in Agepon which acts as a detergent. Treatment time approx. 1 minute.
In order to remove the dissolved stains and to stabilize the positive against future deterioration, it is now placed in a fixing bath for 10 minutes.
The positive is then rinsed in running water of not over 20%C for at least 1 minute.
In order to facilitate the rinsing out of remains of hypo, the positive is treated for approx. 3 minutes in 20°C Hypo Clearing.
The treated positive receives a final rinse for at least 10 minutes at approx. 20°C or colder.
The positive is treated in an Agepon bath for 1 minute.
The positive is dried, away from heat and dust.
Source: E. Weyde. First time printed in “Handbuch der Negativ-Restaurierung”, Bildarchiv Foto Marburg 1977. Unofficial book.
The method builds on reducing the image silver of the discoloured negatives into halogen silver [silver halide] and then re-developing into the original image silver.
On negatives with gelatine emulsion which stand treatment in hydrous [aqueous] solutions.
Can be used on bleached out and/or discoloured gelatine negatives.
Use of oxidized developer which forms yellow oxidation products which may discolour the gelatine in the emulsion (yellow discoloration's wholly or partly). Insufficient development, rinsing of fixer and similar mistakes in preparation (for example dichroic silver).
For the treatment is required:
At least 5 photo trays larger than the size of the photographs which are to be treated
Washing possibility by running water
Cotton wool
Strong light source
Safety equipment
Stopwatch
Copper chloride CuCl2
Citric acid C6H8O7
Glacial acetic acid (98%)
Sodium acetate
Kodak Dektol or Kodak D-72 developer
Agfa Agephon
| Water | 750 g |
| Copper chloride | 125 g |
| Citric acid | 4 g |
| + water up to | 1000 ml |
Special Amidol developer
or
Kodak Dektol developer. Solution to be used: 1 part of concentrated developer for 2 parts of water
or
Kodak D-72 developer. To be dissolved as described on the package. Solution to be used: 1 part of concentrated developer to 2 parts of water.
| Glacial acetic acid (98%) | 7 ml |
| Sodium acetate | 11 g |
| + water up to | 1000 ml |
| Agfa Agephon | 5 ml |
| + water up to | 1000 ml |
Description of state should be made before any form of treatment is started.
The dust is cleaned mechanically off the negative.
Safety duplication.
Test of silver remains by Kodak ST-1. If there are any silver remains present in the emulsion (bad fixing), the picture must be re-fixed and rinsed. The re-fixer takes place in a non-hardening, neutral 20% sodium thiosulphate fixer.
As re-development silver remains may cause destruction of the image silver as the originally not-exposed halogen silver, not removed by the fixer, will be exposed and developed. [Residual silver not fixed out during the original processing will be exposed and developed during this treatment causing irreversible damage to the image.] This will cause an inappropriate blackening of the original.
Bleached out pictures should not be re-fixed, as bleached out image silver “belonging” to the motive [image] will be transferred [removed]. This causes a weakening of the motive [image after redevelopment].
As a consequence of this, bleached out and badly fixed pictures should not be treated in this solution in the first place.
Before the real process starts, one may get an impression of the progress and result of the treatment by first testing all the steps of the process in the form of spot test. The test is made in a corner of the picture and/or in an area less essential to the motive [image], as for example along one of the edges. The result of this test gives only a faint hint of the final result. If, however, problems arise already in this phase one should of course abstain from a further treatment of the picture.
Hereafter, the picture will be treated in the bleaching bath. The copper chloride bleacher has a very limited capacity therefore not too many pictures should be treated in the same bath. The time needed for the bleaching treatment is about 30–60 sec. at 20°C., or until the picture is totally bleached out.
The picture is now washed under running water at about 20°C, for as long as the washing water is “whitish/opalic.”
Now the white silver halogenic [silver halide] picture is exposed in a strong source of light, for example a photo lamp, until the emulsion obtains a faint purple drawing.
The picture is hereafter exposed until the original blackening is obtained in Low pH developer (Amidol) or Kodak Dektol at a developing temperature of 20°C for about 2–3 minutes.
The pH value in the developer is very high (about 10–11). This is why the emulsion of the picture swells violently which can easily be seen. By introducing a stop bath between the developing and the final wash with a pH value of about 4,9 the emulsion is stabilised and at the same time as the developing materials are deteriorated. The time of the treatment is 30 sec. at 20°C.
The picture is given a final careful wash in running water for 30 minutes at 20°C.
The picture is finally dried—with difficulty—between two pieces of strong filter paper for about 12–15 hours, depending on the RH.
MSK 11.08.93
The method builds on reducing the emulsion of the discoloured negative into halogen silver [silver halide] and then redeveloping it into metallic silver.
This can be used for negative materials, on plastic support, which can stand longer treatment in aqueous solutions, such as cellulose nitrates, cellulose acetate and polyester negatives with silver bromide gelatine emulsion.
Bleaching with potassium permanganate can be used with advantage for negatives with strongly damaged image silver. The emulsion can, however easily, be loosened from the support. The method is therefore suited for strongly damaged image silver, but not suited for a weak gelatine.
If you have a weak gelatine one should first try with the copper chloride method.
The method must not be used for negatives with collodion emulsion or negatives with water damage and microbial attacks.
Where the negatives are attacked by sulfide or other attacks which have discoloured the picture forming silver [image silver]. Intensified negatives with such a vigorous bleaching that ordinary redevelopment cannot recreate a normal black/white blackening.
Use of oxidized developer which forms yellow oxidation products which can discolour the gelatine in the emulsion (yellow discolourings wholly or partly). Unsatisfactory development rinsing out of fixer or similar error (for example dichroic silver). Finishing treatment of thin negatives with a chrome intensifier (bleached out or yellow negatives) as well as sulphurous packing materials (for example glassine bags).
For the treatment procedure the following is necessary:
7 or more photo trays twice the size of the negatives which are to be treated.
1–2 washing possibilities where soft and careful rinsing is possible. The negatives must not be left rotated. [The gelatin emulsions will be very delicate so don't let the negatives move around too much either due to manual manipulation or by the rinse water movement.]
Scalpel
Folder
Waterproof tape (3M Scotch Pressure Sensitive Tape, No 853)
Supporting material (for example fixed sheet film)
Cotton wool
Cotton gloves
Metal tongs (which are also used for positive copying)
Strong light source
Fine brushes (for example 00 or 000)
Fume hood, glasses, mask with correct filter equipment choice (if you don't use a fume hood)
Formaldehyde (37% weight dissolution)
Sodium carbonate (monohydrate)
Potassium permanganate
Sodium chloride
Sulphuric acid (concentrated)
Sodium bisulphate
Glacial acetic acid (98%)
Sodium acetate
Amidol, Kodak Dektol or Kodak D-72 developers.
| Water | 500 ml |
| Formaldehyde (37% weight sol.) | 10 ml |
| Sodium carbonate (monohydrate) | 6 g |
| + water up to | 1000 ml |
Stock solution A
| Potassium permanganate (a) | 5 g |
| + water up to | 1000 ml |
Stock solution B
| Cold water | 500 ml |
| Sodium chloride | 75 g |
| Sulphur acid (concentrated) (b) | 16 ml |
| + water up to | 1000 ml |
(a) Potassium permanganate should be completely dissolved.
(b) Always add sulphuric acid gradually to cold water stirring continuously. Thus a development of too strong heat, which could make the water boil, is avoided.
Use gloves and eye protection.
| Sodium acid sulphate [sodium bisulfate] | 10 g |
| + water up to | 1000 ml |
Amidol, special developer
As regards the essential load as an aged gelatine is exposed to through a chemical treatment, it was found right to prescribe this recipe with a low pH-value. [Older gelatin emulsions are exposed to strong stresses during chemical treatment so low pH developer is prescribed.] Thus the gelatine does not work so much, and the risk at the emulsion “lift/swimming” away is easier avoided at the following wash.
| Water 20°C | 650 ml |
| Sodium sulfite (Na2SO3) | 28 g |
| Amidol 2,4-Diaminophenol dihydrochloride (C6H8N2O + 2 HCl) | 6 g |
| Potassium bromide (KBr) | 1,4 g |
| Water up to | 1000 ml |
The developer must be blended immediately before use; only durable for a short while.
or
Kodak Dektol. For ordinary solution dilute with 2 parts water to 1 part conc. developer.
or
Kodak D-72: To be dissolved as indicated on the packet. For ordinary solution is diluted with 2 parts water to 1 part concentrated developer.
| Glacial acetic acid (98%) | 7 ml |
| Sodium acetate | 11 g |
| + water up to | 1000 ml |
| Agfa Agephon | 5 ml |
| + water up to | 1000 ml |
Description of state, etc., is made before any form of treatment is started.
The negative is cleaned mechanically (dust is brushed away etc.) and possibly with water (only base side) and ethanol.
Safety copying/duplication.
Silver remains test with Kodak ST-1. If there are silver remains present in the negative (bad fixing), this has to be re-fixed and rinsed. Re-fixation should happen in a non-tempered, neutral sodium thiosulfate fixer.
Silver remains can by re-development spoil the image as originally non-exposed halogen silver, which is not removed by the fixer, will be exposed and developed. [Residual silver not removed during original processing will be exposed and developed during the process and will spoil the image.] This gives irregular blackening of the original negative. Bleached out negatives should not be re-fixed, as bleached out halogen silver is removed (if it is in the state) which “belongs” to the motive. Thus a weakening of the negative takes place. This type of negative should first be re-developed before re-fixation.
Mounting on support material: Negatives on nitrate, acetate or polyester base have often an antihalation layer on the not emulsion carrying side of the base. This can in some cases be more sensitive to bleaching out and a re-developing process than the emulsion. If this is the case one can avoid that the wet process works on this film by mounting the negative with the emulsion side up on a supporting material of plastic.
A piece of plastic of a certain stiffness and strength (for example a piece of fixed sheet film) is used. It should also be of a size that is larger than the negative which is to be mounted.
The negative is placed on top of the plastic base with the emulsion side up. By waterproof tape the negative is mounted firmly to the underlayer [secondary support], seeing to - with a holder - that all connections fit tightly. The tape is placed over the negative as little as possible (about 1–2 mm) in order to treat so large an area as possible.
Some negatives have holes through the emulsion and base which originate from drying pins or by accidents. These holes should be closed by tape which is placed on the base side of the negative.
Thus the reverse side is protected against water and chemicals and can easily be handled at the same time in the different baths with steel tongs.
Before the real process is started one may have a feeling of the course of the treatment and the result by first testing all process steps in the form of spot-tests. The tests are made in a corner of the negative and/or in an area not essential for the motive [to the purpose of the image] like for example along one of the edges. The result of this test gives only a vague hint about the final result. If, however, problems already arise in this phase one should of course abstain from a further treatment of the negative.
If one disposes of discarded negatives with the same damage, it would be a great advantage to use these instead for spot-test rather than doing this on original negatives. The material and the age of the negatives should, however, be the same, that is come from the series of photos which one wants to treat.
To prevent the gelatine in the photographic emulsion from being dissolved and leaving the base, the negative should first be hardened in a hardening bath containing formaldehyde (which hardens) and sodium carbonate (which “opens” the emulsion, so the formaldehyde can penetrate). [A pH ≥ 5 is also required to remove a proton (H+) from some of the lysine ε-amino groups making them available for reaction with the aldehyde.]
The time of the treatment is 2–3 minutes at 20°C in constant movement. The negatives are rinsed for 5 minutes to remove extra hardening bath.
Hereafter, the negative should be treated in a bleaching bath.
As the stock solutions A and B are very unstable when mixed, they are not mixed until immediately before use in the ratio of 1:1. For the same reason the bleaching bath has only a capacity for the treat of 2–3 negatives. The capacity depends furthermore on the speed of the treatment and on the extent of the damage.
The bleaching bath should kept at a temperature of about 20°C, thus the time of the treatment is about 3–8 minutes in motion.
In the acid solution of potassium permanganate and sodium chloride the discolourings of the emulsion will be transformed into a colourless substance which is soluble in water (namely the bleaching bath). In the same process the image silver will be transformed into whitish silver chloride - it will be re-halogenized. At the same time brown stone (a- labandite dioxide) is formed, which can be observed as red/brown shades over the emulsion. [Manganese dioxide]
The bleached-out but reddish/brown negative is treated in a neutralisation bath.
The negative is treated in agitation until all the brown stone has disappeared and the emulsion is completely whitish.
If all discolourings have not been completely dissolved in the bleaching bath, yellow to reddish and brownish areas may remain on the negative after treatment in the neutralisation bath. This means that the original image silver has not been totally transformed into halogen silver. In this case the image can again be treated in the bleaching bath and afterwards in the neutralisation bath. Another possibility is that the pictures dried and afterwards is bleached and neutralised once more. The latter solution is more gentle to the negative and this is why it is preferred for especially sensitive images.
The negative is now rinsed for 5 minutes at about 20°C after which the whitish negative is exposed to a strong cone of light. This may be sunlight or a photo lamp. The exposure takes place until the emulsion gets a tint of violet or in other words until a “print-out” effect can be observed.
The negative is then developed into original blackening in for example Amidol or Kodak Dektol 1:1 or 1:2. Development is made at a temperature of 20°C. As only image forming silver is in the negative, one cannot overdevelop. On the other hand, underdevelopment should be avoided. The procedure is therefore to develop until the negative does not take more blackening and to continue the development for about 1 minute. This is a way to make sure that the developer works down through the gelatine to develop all the halogen silver.
The pH value in the developer is very high (about 10–11). This is why the gelatine emulsion of the negative swells violently which easily can be seen (or felt - ugh)! If the negative is immediately placed in rinsing water, this will - as well as pressure changes (explanation with osmotic pressure) - see Hendriks from the ICOM Congress in Copenhagen (edit.) produce the result that the emulsion looses attachment to the base! By introducing a stop bath (acid) between the development and the finishing rinse (possibly omit finishing rinse and only rinse in stop bath edit.) it is very possible to prevent the emulsion from loosening from the base in most cases - but experience shows that it cannot be prevented in all cases.
The stop bath should have a pH-value of 4,3–4,4 which is obtained by the indicated recipe. At this value the gelatine swells a minimum. (New information from Kodak and Hendriks indicates that because of the influence of the osmotic pressure a really acidic stop bath should be used - this has, however, to be verified further - edit.)
30 sec. at 20°C.
Plastic base negatives mounted on supporting material are remounted before the final wash. The damp emulsion has swelled and is thus larger than the dry base side. This is why the negative will roll up violently while drying. This is prevented by moistening the base side during the final rinse. By so doing the stress between the two sides will similar be alike, and the final drying will result in a plane negative.
Before the final wash the negative is placed in an Agepon bath which will quickly relax and straighten the negative.
The negative is given a final wash in moderately running water for 20–30 minutes at a max. of 20°C. Here the reaction of the emulsion on the rinsing process has to be constantly observed where a loosening from the base may begin to occur. If this happens, the wash is stopped. The finishing wash possibly can take place after an intervening drying process. If the emulsion is loosened so much that a transportation to another base material is considered necessary, this is done as described in the section on the transportation of emulsions.
It is worth notice, that it is always sensible to have prepared a transfer to another base before this treatment method is used, as sufficient time is thus obtained to save the negative!
At last the negative is dried in a dust-free place without the use of any heat.
MSK 12.08.93
Developer for Bleaching/Redevelopment With Low PH-Value
As regards the essential load as an aged gelatine is exposed to through a chemical treatment, it was found right to prescribe this recipe with a low pH-value. Thus the gelatine does not work so much, and the risk at the emulsion “lift/swimming” away is more easily avoided at the following wash.
| Water 20°C | 650 ml |
| Sodium sulfite (Na2SO3) | 28 g |
| Amidol, 2,4-Diaminophenol dihydrochloride (C6H8N2O + 2 HCl) | 6 g |
| Potassium bromide (KBr) | 1,4 g |
| Water up to | 1000 ml |
The developer must be blended immediately before use and is only durable for a short while.
Amidol is also called: 2,4-Diaminophenol dihydrochloride (C6H8N2O + 2HCL) is bought through Fluka Chemika catalogue 33230.
Jesper Stub Johnsen and Wall
MSK 11.08.93
Thiourea is reasonably anticipated to be a human carcinogen based on sufficient evidence for the carcinogenicity of thiourea in experimental animals (IARC V.7,1974). When administered in the drinking water, thiourea induced thyroid adenomas and carcinomas in rats of both sexes and squamous cell carcinomas of the Zymbal gland in male rats. When administered in the diet, thiourea induced hepatocellular adenomas in rats and hepatomas in rainbow trout. When injected intraperitoneally and administered in drinking water, thiourea induced squamous cell carcinomas and mixed cell sarcomas in the Zymbal gland of rats of both sexes. There are no data available to evaluate the carcinogenicity of thiourea in humans (IARC V.7,1974).
Thiourea occurs as white, lustrous crystals or flaky solids. It is soluble in cold water, ammonium thiocyanate solution, and ethanol and almost insoluble in ether. When heated to decomposition, it emits toxic fumes of nitrogen oxides (Nox) and sulfur oxides (Sox). Thiourea is available in the United States as a 99% pure reagent grade. It may react violently with acrolein.
A recent evaluation indicates that thiourea now is used only in animal glue liquefiers and silver tarnish removers, and that these uses are diminishing. Liquid animal hide glues contain 10–20% thiourea as a liquefying agent. Another report indicates commercial use of thiourea in the production of flame-retardant resins and as a vulcanization accelerator. Thiourea (12%) is also used in a metal cleaner. Formerly, the chemical was used in the production of diazo-type coatings for copy paper as an anti-yellowing agent; in boiler water treatment to remove copper scale; to prevent the appearance of brown stain of hemlock wood; as a silver toning agent in photographic papers; in the synthesis of pharmaceuticals and insecticides; as a catalyst in the isomerization of maleic acid to fumaric acid; in hair preparations; in chelating agents; in dye intermediates; in dry cleaning chemicals; as an antithyroid agent; as a fungicide; as an accelerator of sprouting in dormant tubers; as a weighting agent for silk; as a dye-bath adjuvant of textiles; as a substitute for urea-formaldehyde resins; as a pickling inhibitor and ingredient in plating baths for metals; in the preparation of nonglare mirrors; in the synthesis of sulfathiazole and thiouracil; for the treatment of nylon to prevent running and improve handling properties; and as a reagent for determination of bismuth and selenite ions (IARC V.7,1974).
9th Report on Carcinogens
Revised January 2001
U.S. Department of Health and Human Services
Public Health Service
National Toxicology Program
Pursuant to Section 301(b) (4) of the Public Health Service Act as Amended by Section
262, PL 95–622
http://ehis.niehs.nih.gov/roc/ninth/rahe/thiourea.pdf
Subject: Fischer, fyi
Date: Tue, 09 May 2000 13:47:43 −0400
From: Paul Messier <pm@paulmessier.com>
To: “Doug Nishimura (E-mail)” <dwnpph@ritvax.isc.rit.edu>
Hi Doug: If I'm remembering correctly, you are doing a presentation of the Kent Workshop. If so, you might be interested in the following results. Basically, I treated some “conservation grade” (high alpha cellulose) blotter paper with the Fischer solution and washed it in various ways. The idea was to see if the sulfur remained behind in the paper or remained pretty soluble as expected. Well, as expected, it looks like the thiourea material clears pretty well. Richard Newman at the MFA did the analysis for me, which he actually did at the Fogg. It is his write up that appears below.
All the usual caveats apply…
If you want more details, let me know.
Paul
The sulfur content of pieces of paper washed by several methods was determined by wavelength-dispersive X-ray fluorescence in an electron beam microprobe. Large squares of paper were submitted for analysis.
From each of these an approximately 2 mm × 2 mm square was cut, and three small areas, each about 50 micrometers × 50 micrometers, were analyzed on each of these squares. Below are reported the sulfur peak and background counts, and net counts for the sulfur peak.
Control (no Fischer treatment, no wash)
No wash (Fischer treatment, no wash)
All samples below treated with Fischer
Water wash
Warm water wash
Ammonia wash
Although the actual sulfur content was not determined, the following comments can be made from the results:
1. Samples from all three wash treatments (water, warm water, and contained very small amounts of sulfur, but these levels were below the amount of sulfur present in the control (untreated) sample.
2. The sample that had not been washed had a sulfur content about 50–100 times higher than that of any of the washed samples. This factor was determined simply by comparing the net sulfur peak counts of the washed samples (which varied from 0 to 67) with the net sulfur peak count of the unwashed sample (about 3500–3900).