Topics in Photographic Preservation 1989, Volume 3, Article 3 (pp. 12-21)
Mercuric iodide intensification of silver gelatin negatives produces unstable negatives which over time take on a more or less uniform lemon-yellow coloration accompanied by a loss of contrast. After having found evidence of mercury and silver iodide salts in deteriorated original negatives, the author reproduced the deterioration on modern flexible support negatives and glass plate negatives using accelerated aging techniques. A method of restoration through development produced the return of image contrast, as well as stabilizing the image. The risk of delamination of the binder layer from the glass supports was minimized by controlling the pH of the re-development bath. Measurements were taken of the swelling induced in the gelatin layer by the various procedures.
The conditions of photograph taking at the turn of the century often incited photographers to practice chemical treatments so as to correct the density of their negatives. In fact photographic plates were not very sensitive and light meters and artificial lighting were non-existent. In addition the use of printing out paper known as aristotypes, necessitated high contrast negatives.
When a negative was underexposed the photographer applied a intensification treatment. The mercuric iodide treatment provided somewhat of an advantage in that it was easy to prepare, and allowed for a progressive intensification process that could be stopped when the desired intensity was reached.
However, the image obtained in this manner was instable and prone to yellowing. In order to avoid this alteration, it was considered advisable to redevelop the photograph. Unfortunately it seems that this bath was often forgotten.
So as to better understand the mechanisms of alteration we applied the same treatments both to modern supports and to old glass plate negatives. These were analyzed so that we could develop a method for conservation treatment.
Several analytical techniques were employed to permit us to identify the residual chemical compounds present in the yellowed plates of the collection.
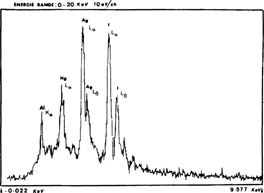
Energie dispersive X-ray spectra for a fragment of a deteriorated plate revealed the presence of silver, iodine and mercury. (fig. 1)

FIG. 1: JEOL JSM-35C -MICROPROBE ORTEC system 5000
MICROPROBE SCANNING ANALYSIS OF A DETERIORATED SAMPLE
These preliminary analyzes confirmed earlier intensification of these negatives with mercury iodide.
We then applied this treatment to silver gelatin negatives, in the form of either glass plates or gray scales on film. Two formulas were used : the first, known as Kirchoff's (5), is a mercuric chloride, sodium thiosulfate, potassium iodide solution. The second, recommended by the Lumière Brothers, is a mercuric iodide, sodium sulfite solution.
A uniform increase in density and a dark green coloration of the image were observed on intensified samples (fig. 2). These negatives were then artificially aged in an oven at 70°C and at different levels of relative humidity (50 % and 90 % RH).
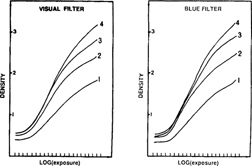
FIG. 2: LUMIERE'S INTENSIFICATION TREATMENT
The color of the sample evolved with time from dark green to pale yellow, irrelative to the intensification formula used. (fig. 3)
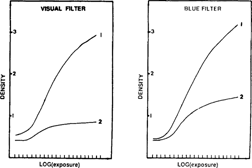
FIG. 3: DENSITY LOSS OF AN INTENSIFIED SAMPLE AFTER AGEING
The density decreased progressively. (fig. 4)
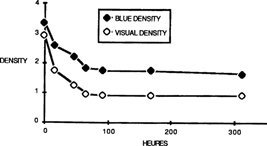
FIG. 4: DENSITY LOSS (at Dmax) OF INTENSIFIED SAMPLES DURING ACCELERATED AGEING AT 90%R.H.
The rate of degradation, after artificial ageing, increases with the duration of intensification treatment. (fig. 5)
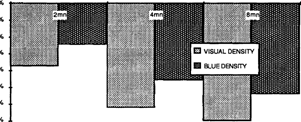
FIG. 5: DENSITY LOSS (at Dmas) AFTER ACCELERATED AGEING OF INTENSIFIED SAMPLES AS A FUNCTION OF INTENSIFICATION TREATMENT DURATION
Humidity accelerated this phenomenon. (fig. 6)
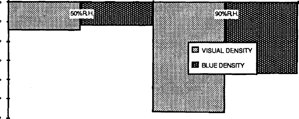
FIG. 6: DENSITY LOSS (at Dmas) OF INTENSIFIED SAMPLES AFTER ACCELERATED AGEING AT 50% AND 90% R.H., 3 DAYS
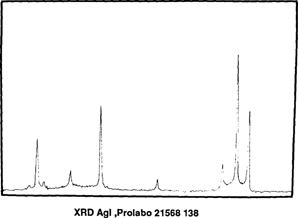
Before, during and after accelerated ageing we analyzed samples by X-Ray Diffraction Spectrometry (TABLE 1). After mercuric iodide treatment we found mercurous iodide and silver iodide on the samples (fig. 7). During accelerated ageing mercurous iodide disappeared progressively (fig. 8). After 15 days at 70°C and 90 % (percent) RH only traces of mercurous iodide remained while the amount of silver iodide was very significant (fig. 9). An identical spectrum was obtained from a deteriorating glass plate in the collection (fig. 10).
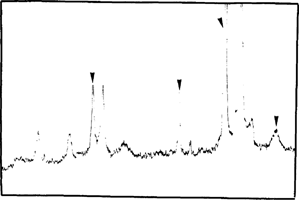
FIG. 7: XRD ANALYSIS-NEGATIVE AFTER INTENSIFICATION TREATMENT AgI, Hg2I2 (▿)
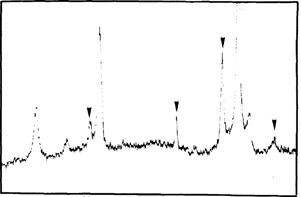
FIG. 8: XRD ANALYSIS-NEGATIVE DURING ACCELERATED AGEING AgI; Hg2I2 (▿)
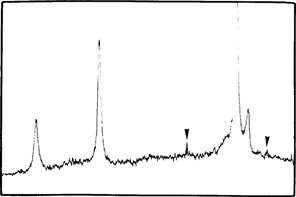
FIG. 9: XRD ANALYSIS-NEGATIVE AFTER ACCELERATED AGEING AgI; Hg2I2 (▿)
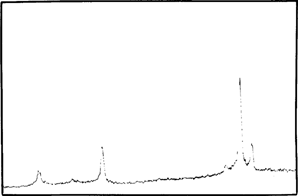
FIG. 10: XRD ANALYSIS-DETERIORATED NEGATIVE FROM A COLLECTION AGl
XRD ANALYSIS
| Agl | Hg2I2 | Agl | fig.7 | fig.8 | fig.9 | fig.10 | |||||||
| ASTM | ASTM | prolat | |||||||||||
| d(Å) | l/l | d(Å) | l/l | d(Å) | I | d(Å) | I | d(Å) | I | d(Å) | I | d(Å) | I |
| 4.54 | 3 | 4.58 | 3a | 4.54 | 4a | 4.58 | |||||||
| 3.98 | 6 | 3.98 | 6 | 3.98 | 4b | 3.94 | 4b | 3.96 | 4b | 3.99 | 4 | ||
| 3.75 | 10 | 3.75 | 10 | 3.75 | 10b | 3.73 | 10b | 3.73 | 10b | 3.78 | 10 | ||
| 3.51 | 4 | 3.51 | 2 | 3.51 | 4b | 3.49 | 4b | 3.54 | 3 | ||||
| 3.49 | 10 | 3.51 | 10a | 3.49 | 10a | ||||||||
| 2.91 | 3 | 2.94 | 5a | 2.95 | 4a | 3.04 | |||||||
| 2.73 | 2 | 2.73 | 1 | ||||||||||
| 2.47 | 3 | 2.45 | 3a | 2.45 | 3a | ||||||||
| 2.30 | 9 | 2.30 | 6 | 2.31 | 6b | 2.28 | 8b | 2.29 | 7b | 2.30 | 4 | ||
| 2.23 | 5 | 2.25 | 5a | 2.23 | 3a | ||||||||
| 2.12 | 3 | 2.12 | 2 | 2.11 | 3b | 2.10 | 3b | 2.11 | 2.13 | 1 | |||
| 1.96 | 5 | 1.96 | 4 | 1.96 | 4b | 1.96 | 4b | 1.95 | 3b | 1.96 | 2 |
When a negative is immersed in a intensifying solution, the silver that makes up the image is partially transformed into silver iodide which is yellow, and into a dark green mixture of mercury and mercurous iodide(1).
These compounds notably increase the density of the image. However, the mercurous iodide is not stable : it decomposes into mercury and mercuric iodide with heat and light (6). The mercuric iodide thus formed can once again react with the silver:
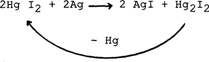
This cycle probably continues to renew itself until the depletion of one of the compounds:silver or mercuric iodide.
Since deterioration results from the transformation of silver into silver iodide, the image just has to be redeveloped in order to reverse the process by reduction of the silver iodide into metallic silver.
Several techniques for the conservation of these negatives have already been proposed (3). We have not tried the bleach and redevelopment method used by Mr JOHNSEN (4).
We first tested the effect of dilute Dektol (1 : 1) on glass plates as well as on film, intensified then artificially aged.
So as to improve the action of the developer it proved indispensable to expose the image during treatment to a strong light source (solar light, xenon lamp). Without this exposure it is difficult to obtain correct densities and the negative retains a brown coloration.
Good results were obtained, using this method, for modern films. The film regains its neutral tone.
For glass plates results are less conclusive, the plate is intensified after having been immersed in the developer. Unfortunately there is a tendency for the gelatin to frill from the support during rinsing. The severe variation of the pH value between the developer bath and the rinse water seems to be the principal cause of this alteration.
It is important to note that these first conservation attempts were made on artificially aged negatives on glass plates, but the gelatin undergoing these severe conditions is made fragilize to a much greater extent than it would have had it undergone natural ageing.
We treated in this way turn-of-the-century negatives in the collection presenting this type of yellowing.
Although the gelatin sometimes tends to frill at the edges, it remains securely adhered to the plate.
We sought nevertheless to find an active developer with a pH value close to neutral.
The formula proposed by HAIST (2) is particularly well adapted to the development of silver iodide emulsions. Moreover it can be used for a wide range of pH values.
The ion complex developer is active on artificially altered gray scales (fig. 11). The characteristic curve was more or less restored.
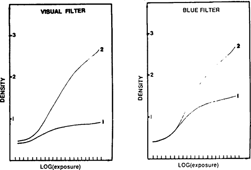
FIG. 11: RESTORATION THROUGH DEVELOPMENT
Analysis by X-Ray Diffraction Spectrometry revealed the disappearance of silver iodide, transformed into metallic silver by the developer.
We applied this treatment to several glass plate negatives from the turn of the century. The image was intensified, the yellow color disappeared, and there was no separation of the image layer from the glass support during washing.
During the conservation treatment of the glass plates, we made a point of closely observing the swelling of the gelatin in the developer and the rinse baths. Our measurements indicate that by using a developer with a close -to -neutral pH value we can limit the swelling of the gelatin during treatment (fig. 12).
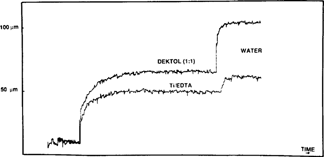
FIG. 12: SWELLING OF THE GELATIN LAYER DURING TREATMENT (glass plate negative)
To assure the long-term innocuousness of this conservation treatment, the images treated with the developer were placed in an aging oven. Since thermo-hygrometric conditions were severe. After 15 days at 70°C and 90 % R.H. no alteration was visually discernible. The density measurement with a Visual Filter indicate a variation in density of about 5 % and the density measurement with the Blue Filter revealed an 8 % variation for maximum densities.
The experiment was repeated on Dektol-treated films; the results are similar.
This study confirms the instability of images that have undergone mercuric iodide intensification. Residual products slowly oxidize the silver to form silver iodide, giving a yellow color to the image. This alteration seems to be stabilized once all of one reagent has been transformed.
The use of a Titane-Ethylendiaminetetraacetic salt developer for the conservation treatment of these negatives has proved efficient in that no significant long term alterations were produced.
The possibility of working with a close to neutral pH value has led us to recommend this technique.
The generalized use of such chemical conservation treatments must, however, be avoided, especially since quite often a simple duplication is all it takes to restore the contrasts of the phototype if the alteration is a uniform one.
(1) Francois (M.) Sur la couleur de l'iodure mercureux amorphe. In: Journal de Pharmacie et de Chimie, 6, 6, 1897, pp. 529–533.
(2) Haist et al - In: Photographic Engineering, déc. 1956, pp. 182–189. cf.: Glafkides (P.) - Chimie et Physique photographiques. 5e éd., Tome 1. Paris: Editions de l'Usine Nouvelle, 1987, p.122.
(3) Hansch (M.) - Frühe Photographien ihre Technik and Restaurierung; Uberherrn: Kabinett-Verlag Uwe Scheid, 1985.
(4) Johnsen (J.S.) - The Treatment of discoloured glass plate and cellulose nitrate negatives. In: Preprints de la 10éme conférence anniversaire de l'Institut of Paper Conservation (I.P.C.), Oxford, Grande-Bretagne, 14–18 avril 1986, D27–D29.
(5) Londe (A.) - Aide mémoire de la photographie. Paris: J.M. Baillière et fils, 1897.
(6) Varet (R.) - Recherches sur les sels de mercure. In: Annales de Chimie et de Physique, septirème série, 8, 1896, pp. 79–141.
LUMIERE'S FORMULA |
|
| WATER | 1000 ml |
| SODIUM SULFITE | 100 g |
| MERCURIC IODIDE | 10 g |
KIRCHOFF'S FORMULA |
|
| WATER | 800 ml |
| MERCURIC BICHLORIDE | 10 g |
| Then add: | |
| WATER | 1000 ml |
| POTASSIUM IODIDE | 25 g |
A red precipitate forms which then dissolve in the excess reagent, then add:
| SODIUM THIOSULFATE | 1g |
The negative is gradually intensified, then washed.
During the development the samples were exposed at several different times to the light source to improve the action of the develop per. After 5 to 10 mn at 24°C the negative acquires a neutral tonality. It was then washed for 10 mn in distilled water.
HAIST'S develop per:
| Titanium trichloride 20% solution | 75 ml |
| Na4EDTA | 100 g |
| Sodium acetate | 20 g |
| Potassium bromide | 4 g |
| Distilled water for | 1000 ml |
When we prepared this solution its pH was found to be around 6.5. However it is possible to modify it by adding hydrochloric acid. This develop bath can not be conserved.
DEKTOL developer:
| Commercial brand diluted | (1:1) |