Topics in Photographic Preservation 1989, Volume 3, Article 16 (pp. 135-150)
This article presents initial findings in an ongoing investigation of the Collodion Process on Glass. The emphasis is on the role of varnishes with respect to wet plate negatives, and the research is undeniably at a very early and formative stage. Nevertheless, coating thickness and varnish identification procedures in use at this point in the study, recent data, and some some of the longer term objectives of the research are discussed.
The choice of collodion wet plate negatives as a fitting subject for study was motivated in part by the tremendous variations in their physical appearance, much of which can be attributed to the hand crafted nature and wealth of process variables at the photographer's discretion. Along with the expected scratches, abrasion and glass breakage caused by handling abuses, collodion plates can exhibit cracked coatings, flaking, reticulation, discoloration or stains, fading, and tackiness under certain environmental conditions. In extreme cases the coatings become viscous to the the point where the silver image particles begin to migrate as the coatings reflow. Under these conditions entrapment of foreign particles (e.g., paper fibers of enclosure envelopes) also occurs. Fortunately, these severely degraded plates have been encountered in less than 1% of the collection holdings examined so far. Still, are these samples a glimpse of the future for the entire population or hopefully just isolated incidents of poor craftsmanship? Collodion is, of course, cellulose nitrate so the well documented concerns about the chemical stability of this material can easily serve as a basic hypothesis for chemically degraded collodion plates. However, the thickness data presented in this report demonstrates how thin the collodion binder actually is, and other considerations regarding deterioration mechanisms need to be evaluated. A comprehensive picture would include the following subjects:
This is a vast area to cover, and overlap between these categories is likely. However, a decision was made to concentrate primarily on item 1, the role of the varnish layer. The justification for this is threefold. First, the classical role of varnishes, aside from their decorative properties, is as a protective barrier to prevent the chemical and physical deterioration of the coated surface. Since the collodion image is protected on one side by glass the chemical and vapor barrier properties of differing varnish compositions may well be a significant rate governing factor to some chemical reactions taking place in the image layer. Second, Collodion Era photographers were trying to achieve a significant number of handling properties in their overcoat protection scheme. They desired various physical attributes such as hardness without brittleness, clarity, high temperature stability (to survive sunlight contact printing operations without blocking to the print paper), ability to accept retouching, and ease of cleaning. Consequently, nineteenth century photographic journals suggest as much if not more variability in varnishing practices compared to the image forming steps. One fundamental difference in varnish application existed in the craft; use of a recipe high in alcohol content which necessitated heating the glass plate to about 50°C versus formulas using chloroform or benzene which could be successfully applied at ambient temperature. Third, even under ideal coating conditions the collodion binder has a relatively low adhesion to the glass substrate. Thus, the adhesion of the overcoat to the silver-collodion interface and basic mechanical properties such as thermal expansion coefficient may determine whether the varnish will act as a successful consolidant or adversely stress the collodion binder in a manner which hastens cracking and flaking. The longer term prospects for research on the collodion process will hopefully include the following objectives:
Thickness data is being compiled using a Leitz Component Measuring Microscope. This microscope has precision digital read-out gauges in X,Y, and Z planes capable of measurement repeatability within ± 0.3 microns. A 50x, .60 N.A. objective lens and monochromatic light source is used in a reflection mode. With a 10x eyepiece the magnification of the sample to the user's eye becomes 500x. Thickness profiling is then only a matter of focusing on the top and bottom surface planes of the sample, and recording the digital gauge displacement. When focusing through a coating the index of refraction must be accounted for. However, it was quickly ascertained that collodion plates have enough pinholes and scratches such that in almost all samples various coating defects allow the layers to be observed with air as the only optical medium. On the microscopic level the coating fracture often leaves a small ledge at the silver image surface to establish the thickness of the collodion binder independently of the total thickness. The exposed glass surface is the reference lower plane and the varnish layer is then measured by focusing on the minute abrasions and inclusions which locate its top surface. The method is non-invasive and no physical contact with the collodion coating surface occurs.
A non-destructive varnish identification procedure was also desired and one that would be reasonably time efficient. Fourier Transform Infrared Spectroscopy seemed an appropriate place to start, at least as a means to sort the basic natural resin constituents in the varnish formulas. FTIR spectroscopy has previously been applied to the identification of natural resins and mixtures of these resins commonly found in furniture finishes1. Nineteenth century photographic literature indicates that collodion plates were commonly varnished with the same types of resins; shellac, sandarac, mastic, copal, and dammar, among others. It was hoped that the technique would be completely non-destructive by relying on the IR reflection component from the collodion plate. IR transmission mode was unworkable due to the absorption of the glass substrate. Reflection mode sampling also turned out to be impractical because the multiple interfaces of varnish, silver, collodion and glass interfered with the IR reflectance so that a clean sample spectrum could not be achieved. The method finally adopted was to use the Cygnus 100 FTIR Spectrometer fitted with a Spectra Tech IR-PlanTM Microscope Accessory which enables an FTIR scan of microscopic samples. By using diamond window optics rather than more conventional potassium bromide pellets, microgram samples of varnish can be judiciously removed from the collodion plates and prepared for analysis in an expedient manner. Microscopic samples and straight-forward means of preparation are the main advantages of the method. A disadvantage is the fact that samples must be discarded whereas KBR pellet dispersions can be saved for future reference. Another disadvantage is that the spectra tend to be noisier and the baseline distortion more prevalent owing to lack of uniform sample dispersion. However, baseline correction, smoothing, and deconvolution software can be invoked when necessary. Also, the diamond optic becomes IR absorbing at wavenumbers less than approximately 650 cm-1, but this is not too critical since the natural resins on collodion plates give substantial IR signature between 1750 and 700 cm-1. Viewing under a stereo microscope at 30 to 50X magnification, a tungsten carbide needle is used to carefully collect the varnish sample. By observing that the silver image beneath is not being disturbed it was found that the varnish can be successfully isolated from the metallic particles and the cellulose nitrate. The sample is then dispersed between the two mating halves of the cleaved synthetic diamond by finger tightening three spring loaded shoulder screws. Finally, the diamond optic halves are separated, and the one to which the majority of the sample adheres is then placed on the IR microscope stage. The IR signal is tuned optimally by adjusting the optical path elements of the beam, and a sample scan and background reference scan are run. The total procedure takes about 20 minutes. Carefully managed, the microscopic extract leaves the silver image intact, and the varnish removal is so small that no visible damage is noticeable to the unaided eye. It may also be stated that the hand coated plates have literally hundreds of coating blemishes which are of a larger dimension. Nevertheless, as a matter of conscientious practice the sampling procedure is confined to less critical areas of the plate outside the useful printing borders of the collodion image.
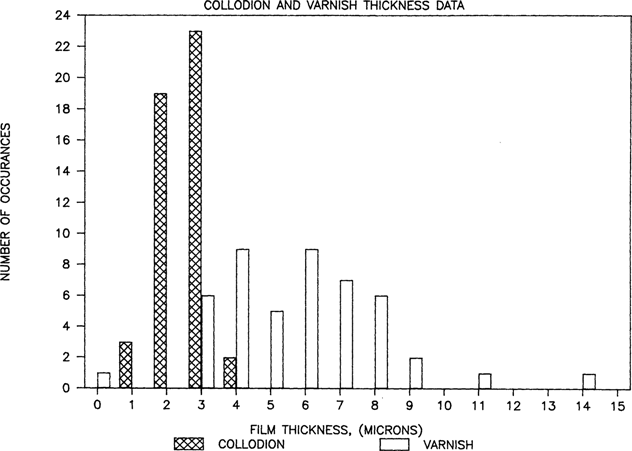
Collodion glass plates have thus far been obtained for research examination at the Conservation Analytical Laboratory from two sources at the Smithsonian Institution in Washington, D.C.; the Bureau of American Ethnology (BAE) collection at the National Anthropological Archives (identified in this report as NAA samples) and the Meserve collection at the National Portrait Gallery (NPG samples). There are approximately 300 6.5 × 8.5 inch NAA collodion plates and over 5000 NPG images cut down to single CDV size from larger multiple exposure plates. The thickness data for 47 plates is available in Figure 1. Twenty-one are NPG samples. Since no significant differences were found between the collections the data is presented as a single population. Average plate thickness for each sample was plotted. Varnish and collodion flow marks were often 20 to 50 microns thick along edges of collodion plates, but such areas were excluded from the average coating thickness determination. Within the functional image areas, varnish thickness and collodion thickness ranges were typically 2 to 1, occasionally 3 to 1. For example, a thin region of collodion might be 2 microns while a thicker part on the same plate might reach 4 microns. Similarly, a varnish coating might be thin at 4 microns, thick at 11 microns, while averaging 7microns. The change was generally a gradual transition across the plate, not localized regions, so thickness averages are usually very close to the midpoint of the range. Although the microscope could achieve slightly higher precision, data has been reported to the nearest whole micron value. The collodion thickness value must be clarified at this point. The value on the graph is really the collodion plus silver thickness since the silver layer was used to define the bottom of the varnish. Careful focus through the silver containing region indicates that the silver particles occupy at least 1 micron thickness in the coating. Therefore, when collodion layers of one micron are reported it is fair to describe the collodion as a dispersed adhesive bonding the silver to the glass rather than a discreet layer. The silver structure is quite unique and electron microscopy studies are in order, as this subject exceeds the limits of light microscopy.

FIGURE 1
Only wavenumbers less than 2000 cm-1 are shown since the natural resins likely to be encountered on collodion plates have no distinguishing features at higher wavenumbers. NAA plates 3569a and 3579c were made in 1858 and 1867 respectively, and their FTIR spectra are representative results which can be obtained from plates with reasonable chemical stability. In this instance, NAA3569a varnish displays a strong signature indicating predominantly shellac resin while NAA3579c shows characteristic peaks similar to sandarac (See Figures 2 and 3). Reference spectra for shellac and sandarac are also plotted. They were prepared by the same diamond optic technique previously discussed. To date, only a small number of plates have been measured so more analysis is required before findings can emerge about common types of varnish. However, it is reassuring that shellac and sandarac have been identified so early on in the investigation. In a review of 46 varnish formulas for glass plate negatives from photographic journals spanning the collodion era, shellac, sandarac, or mixtures with these resins as main ingredients were cited 30 times. Benzoin was cited 4 times, copal 3, dammar 2, amber 2, and India rubber once. Other miscellaneous natural resins accounted for the remainder.
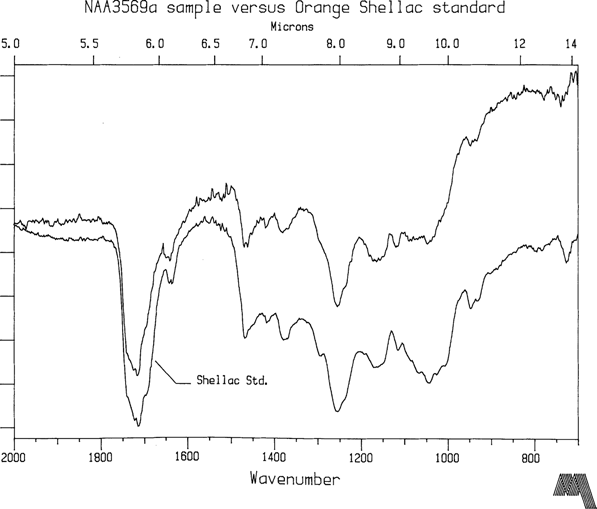
FIGURE 2
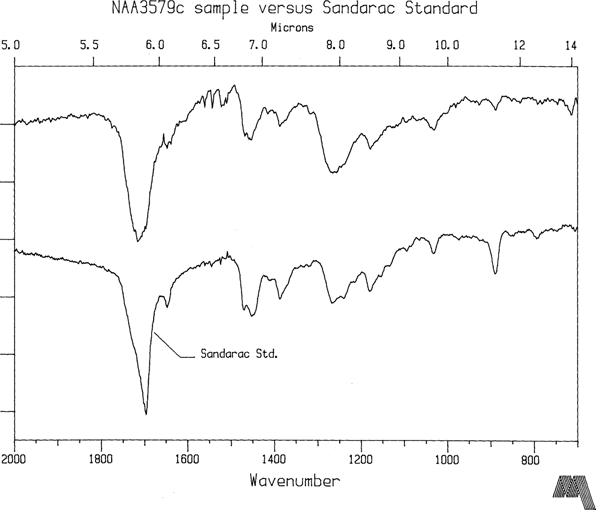
FIGURE 3
NPG plates 5257 and 2575 are two of the most highly degraded coatings in the Meserve collection and belong to that <1% group described earlier in this article. They are Bradio Studio plates as are virtually all the plates in the Meserve collection. Furthermore, collection provenance suggests that these samples were not subjected to environmental conditions significantly different from the group as a whole. NPG2575 is an unidentified sitter so the information content of the two images cannot confirm that they were made by the same photographer or were part of the same batch. Yet the FTIR spectra of both plates is essentially the same (see Figure 4) indicating that they are of the same composition. However, the dominant peak at 1564 cm-1 is not associated with natural resin while the carbonyl groups, 1725 to 1705 cm-1, typically forming a dominant peak in natural resin spectra, give a surprizingly low signal. Fortuitously, some clumps were located in the coatings, larger than 40 micron diameter. Their appearance suggested coating material which had not fully dissolved into solvent solution. Believing that these clumps might shed some light on original constituents in the varnish formula, samples were extracted from them and analysed. The first clump sample is shown in Figure 5. The IR signature has the familiar qualities of a natural resin, especially shellac, while the 1564 cm-1 peak is present at a much smaller signal strength. The second clump extract (Fig. 6) taken from NPG5257 is definitely not a natural resin. The peaks at 1655 cm-1 and 1544 cm-1 indicate proteins. This particle is albumen or gelatin. Both albumen and gelatin have an FTIR signature nearly identical to this plot2, and the FTIR method does not really allow for it to be narrowed down any further than that, especially on an aged sample. However, if this is truly a coating constituent, then the implication is that gelatin or albumen was used on this plate as a subbing or interlayer. On the other hand, it is equally plausible that the clump may have been an albumen particle which transferred and blocked to the plate during a contact printing session. The lack of a clear protein peak in the varnish coating sample spectra tends to support the latter conclusion.
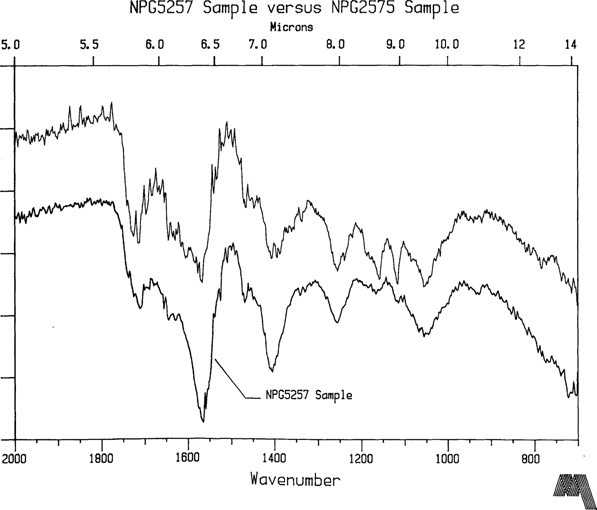
FIGURE 4
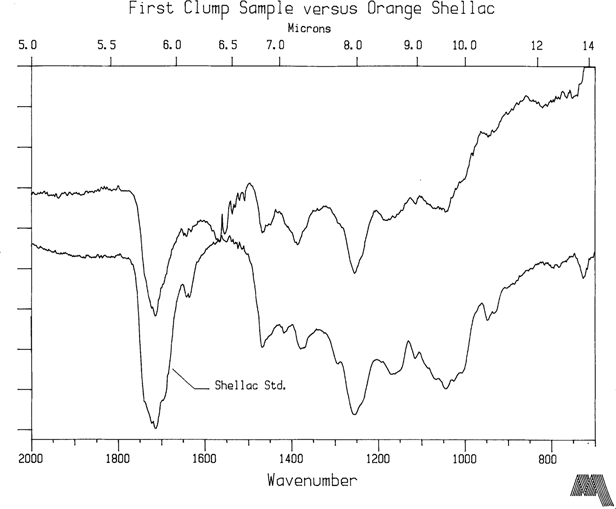
FIGURE 5
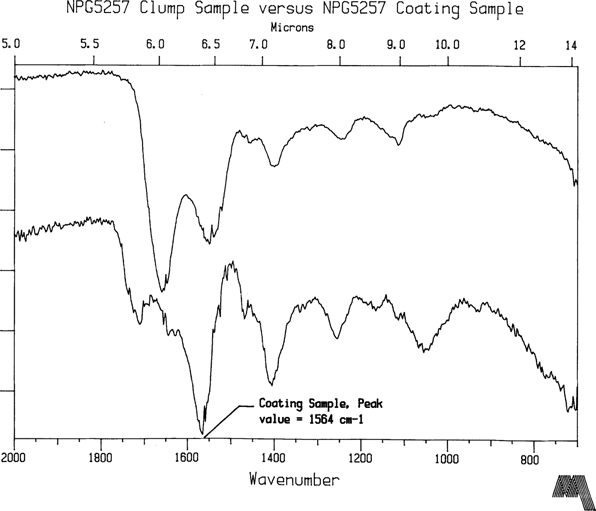
FIGURE 6
Finally, cellulose nitrate was prepared by coating some Collodion USP on glass and sampled as usual. Since the NPG plates have reflowed significantly, it was possible that the varnish sample was contaminated with collodion. Figure 7 illustrates that cellulose nitrate in good condition has strong peaks at about 1660, 1280, 1070, and 840 cm-1. None show in the NPG varnish sample spectra. At this time, systematically degraded samples of cellulose nitrate have not yet been made for reference, and these samples might show shifts in peak location. It seems very likely that the strong peak at 1564 cm-1 is associated with nitrogen containing organic groups. For example, An NO2 out-of-phase stretch absorbs near 1550 cm-1 for R-NO2 groups, and the wavenumber can be raised when electronegative atoms such as Clorine or Oxygen are attached. The fact that the largest peak coincides with a strong nitrogen correlation region in IR spectra certainly implicates classical cellulose nitrate decomposition. The overcoat material may then be breaking down in an additional chemical reaction with the by-products of the collodion deterioration. However, a serious question in this hypothesis still remains. Why is the larger volume of varnish reacting so completely with nitrogen related compounds from an apparently smaller volume of cellulose nitrate such that no starting materials identify themselves in the FTIR data? Is it possible that albumen, gelatin, or possibly normal collodion was present as a third constituent in the varnishing technique?
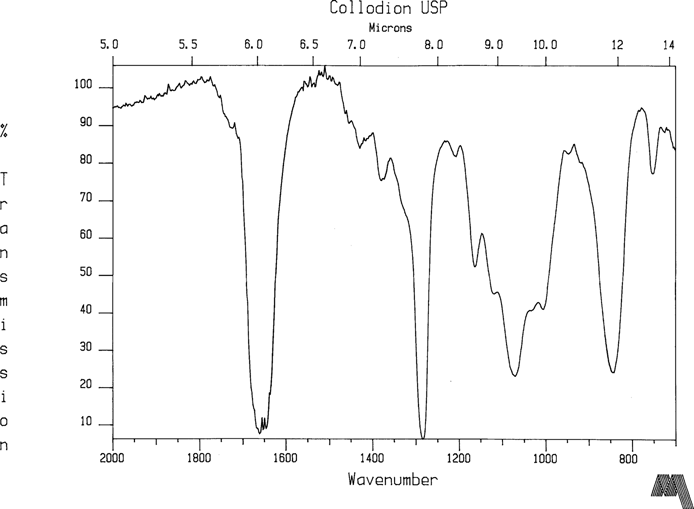
FIGURE 7
Thickness measurements from 47 plates indicate that the the silver collodion image is typically between 2 and 3 microns while the varnish overcoat averages 5 to 6 microns in thickness. Two varnish samples measured significantly thicker, 11 and 14 microns. One NAA plate from 1858 was not varnished. The two collections which have been sampled so far are very regional in nature, though. All studios were in Washington or New York. The sample size is also too small at this time to extrapolate beyond the two study collections. More sampling from a wider range of collections will be required before the data can be safely claimed to represent the general practice of collodion photography in America.
FTIR spectra from the first plates which have been examined has revealed that sandarac and shellac are easily identifiable constituents in varnishes used on collodion glass plates. This is consistent with nineteenth century literature. As the work progresses, a reference library of natural resin spectra is being generated or acquired in order to aid in the identification procedure. Seriously degraded collodion plates exhibiting signs that they are or were at one time tacky to the point of dissolution present a more complex picture. NPG 2575 and 5257 were selected as initial FTIR candidates precisely because they are in such substantially poorer condition than the overwhelming majority of the plates. One of the original varnish constituents was probably shellac, isolated by the analysis of a resin clump on one plate which had not fully dissolved. Otherwise, its identity is masked when samples are taken from the overcoat layer in its present condition.
Tackiness of collodion plates bears final comment. None of the recently examined plates were found to be tacky even though it is clear that some were at one time. For example, NPG 2575 and NPG5257 were obviously very sticky at one time because the horrendous level of paper fibers embedded in them is a stark testimony. The Museum Support Center building where the Conservation Analytical Laboratory is located maintains a constant 70°F, 50% R.H. level. Acclimated to this condition these NPG samples now show only a slight tendency to block to their enclosure envelopes. Notes made when the the Meserve collection was accessioned by the Smithsonian about 1981 also report various plates to be tacky. Several plates with specific notation were spot checked to verify the original remark. None were found to be tacky. It is very probable that the condition can be brought on again merely by raising the temperature and/or humidity to some level typically encountered in non-air conditioned storage locations. PH buffered enclosure envelopes are small consolation to these plates if temperature and humidity are not controlled.
1). Derrick, Michele, “Fourier Transform Infrared Spectral Analysis of Natural Resins Used in Furniture Finishes”, Postprints of papers presented at the Wooden Artifacts Group, Specialty Session, A.I.C. Annual Meeting, New Orleans, LA., June 5, 1988
2). The Sadtler Standard Spectra, Sadtler Research Laboratories, Philadelphia, PA., 1965
* Research Photographic Scientist, CAL/MSC, Smithsonian Institution