Topics in Photographic Preservation 1991, Volume 4, Article 12 (pp. 156-160)
In 1988, we first discussed the use of alkali metal borohydride compounds as a method for intensification of early printing-out papers, and other faded silver-based photographic materials. We still feel that we cannot endorse its use as a legitimate treatment option because of unpredictable results such as emulsion blistering or roughening, spotty or blotchy effects, color changes possibly different from the original, and the unknown future stability of treated prints.
When effective, the process is simple and immediate. Dilute aqueous solutions of sodium or potassium borohydride are applied by brushing or immersion. The paper base whitens from the bleaching action of the borohydride anion as the image darkens, presumably from the reduction of the oxidized silver compounds back to silver metal. Surface phenomena such as silver mirroring are not affected, and the treatment seems to be effective in varying degrees on all silver processes. Albumen prints are more resistant, however, and more prone to problems.
Recently, borohydride treatments and other chemical methods are being used for the intensification of vintage photographs to a degree not previously thought possible. Thus the potential exists for enhancing a faded image to an extent that it might appear to be genuinely pristine. This has naturally caused consternation among photographic print collectors, curators, and dealers. Obviously it would be highly desirable to find ways of detecting image enhancement, and also to be able to better predict treatment effectiveness and long term stability of treated photographs. These factors could have considerable impact on the market value of some photographs, and collectors as well as museums should be aware that treatment history might affect the value and condition of potential acquisitions.
To this end, we have most recently been attempting to study borohydride enhancement using Transmission Electron Microscopy (TEM). The feeling was that if we could actually observe the silver grains by TEM in a given emulsion, we might see significant differences in silver grain structure with respect to particle size, shape and/or quantity in treated and untreated albumen photographs, and that this might eventually lead to answers for some of the concerns discussed above.
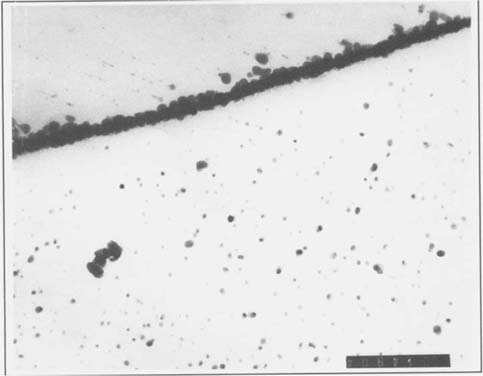
In order to study photographic emulsions by TEM, small samples must be mounted in an appropriate resin, and sectioned so that the sample is thin enough to allow electrons to pass through the mounting resin, emulsion, and paper support, but not through the much denser image silver or silver salt particles. Our initial TEM experiment involved a borohydride treated albumen print. A specimen of this print was mounted in Ward's Bio-plastic liquid casting plastic and sectioned to a specimen thickness of 70 nanometers using an ultramicrotome fitted with a materials analysis diamond knife. The thin sections were studied and photographed using a Phillips 301 Transmission Electron Microscope, operated at 80 KV. Figure one shows a specimen taken from an area of maximum image density, at a magnification of 91,000 X. The silver particles are clearly defined, and these initial results encouraged us to search for changes in the grain structure of other borohydride treated specimens.
Several faded albumen prints were obtained and specimens were taken from adjacent areas before and after treatment, and mounted and sectioned as before. A comparison of the silver particles in these specimens revealed that they were quite different than those observed in the albumen print studied in the first experiment. The individual grains were considerably smaller and less well defined than in the first sample. Figures two and three illustrate before and after treatment samples, respectively, at 91,000 X. It should be noted that this was a print that became much more intense with borohydride treatment. Even though we were expecting to see a few more and/or larger grains in the treated samples, we found that there were greater variations between areas within each emulsion sample than between treated and untreated prints. It may be possible in the future to distinguish subtle differences by using computer assisted statistical analysis of particle size and distribution.
Although these studies are at a very early stage, we do find a few points of interest.
1.) We observed heavy deposits of what appears to be silver at or near the emulsion paper interface. The silver particles found here seemed larger and greater in number than in any other part of the emulsion. It may be that due to the thinness of the albumen layer, this silver could form a primary part of the image.
2.) The grain sizes and concentrations varied considerably between the various albumen prints we have studied. There is a large difference between the grains seen in the first figure and the following pair, even though all the specimens were taken from maximum density areas of albumen prints, and photographed at the same magnification (91,000 X). X-ray fluorescence analyses of the prints studied confirmed that while all of the prints sampled were silver, the print in figure one had been gold toned, while the others were not. Further efforts in this direction might allow us to see if this apparent increase in grain size with gold toning represents a consistent pattern.
We plan to continue our TEM studies using gelatin developing-out papers. The much larger grain size should allow us to more easily observe the physical effects of image enhancement and possibly even enable us to speculate on long term aging characteristics of treated prints.

Figure 1
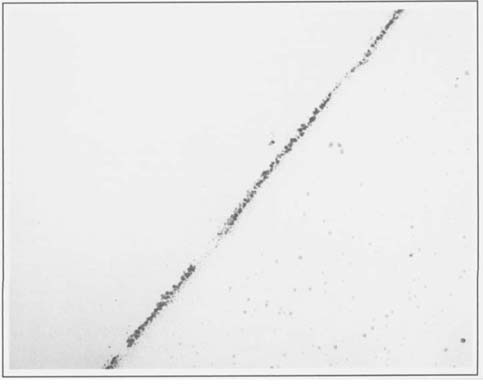
Figure 2
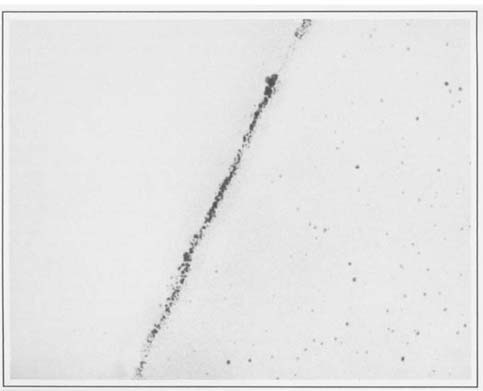
Figure 3
Feldman, L.H.; “Discoloration of Black and White Photographic Prints”, Reprinted from the Journal of Applied Photographic Engineering, Volume 7, Number 1, February 1981, copyright 1981, Society of Photographic Scientists and Engineers.
Haist, G. Modern Photographic Processing, Volume 2, John Wiley and Sons, New York, 1979.
Hendricks, K.B.; “Experiments on the Restoration of Discolored Black and White Photographs in Chemical Solutions”, Society of Photographic Scientists and Engineers: Transcript Summaries, Springfield, VA. SPSE, 1985.
Hendricks, K.B. and Ross, L.; “The Restoration of Discolored Black-and-White Photographic Images in Chemical Solutions.” The American Institute for Conservation of Historic and Artistic Works Preprints, Sixteenth Annual Meeting, New Orleans, LA, 1988.
Stodulski, L.P., Baas, V., and Severson, D.: “A Preliminary Investigation into the Effects of Aqueous Sodium Borohydride Solution on Silver-Based Photographic Materials.” Topics in Photographic Preservation, AIC/PMG, 1989.
Reilly, J.M., Care and Identification of 19th - Century Photographic Prints, Kodak Publication G-2S, Rochester, N.Y. Eastman Kodak Co., 1986.