Topics in Photographic Preservation 1991, Volume 4, Article 15 (pp. 170-178)
Cracking is a physical attribute of albumen photographs which is important from both an aesthetic and conservation standpoint. The cause of cracking has often been linked to excessive physical manipulations of albumen prints. Pressures exerted by calendaring and mounting presses, combined with careless handling, certainly exacerbate albumen's tendency to crack. However, such externally applied manipulations probably exploit stress which is inherent to the albumen layer itself. It is possible to explain the origin of such stress by examining the interrelations and structures of the involved protein molecules.
During the fabrication of albumen photographs, individual protein molecules form a network of protein aggregates. Prior to aggregation and subsequent gel formation, the proteins exist in an aqueous solution containing 87% water (Powrie 1973). Virtually all of this water is lost to evaporation during the initial drying of the newly manufactured albumen print. A “dried-in” stress results as the protein aggregates become immobilized and are thereby no longer physically able to compensate for the volume of water lost to evaporation.
Another possible source of stress may be found within individual protein molecules. The processing of the egg white proteins for the manufacture of albumen photographs causes the involved proteins to become denatured. A denatured protein molecule no longer retains its compact, native conformation of low potential energy. For the egg white proteins, denaturation means that the original globular conformations of single protein molecules are altered to create more linear, less intertwined, molecules of higher potential energy. A stress originating within the molecule may result as the denatured protein of high potential energy gradually reverts to a more compact state of lower potential energy.
As illustrated by the following scanning electron photomicrographs, water has a dramatic effect on the albumen binder. The notable increase in cracking after water immersion implies that a general shrinkage of the albumen layer, relative to the paper base has, taken place. In a laminate system, such as a photograph, the shrinkage of one layer relative to another causes curling. If this curling is restrained, cracking can be the result. Therefore, shrinkage of the protein layer can lead to the propagation of existing cracks and the creation of new cracks.
Exposure to water and high humidity have been linked to the shrinkage of other protein systems (Calhoun and Leister 1959; Mecklenburg 1988; Karpowicz 1989). The partial recovery of dried-in stress imparted during the initial drying of protein systems is often attributed to the effects of water. A partial release of stress would result in a permanent, non-recoverable, loss of linear dimension across the plane of the albumen layer.
It is also possible that water has the effect of “shrinking” individual protein molecules. As already mentioned, a freshly made albumen binder will contain a high proportion of denatured proteins. Liquid water and cycles of elevated humidity could cause the more linear, denatured proteins to revert to more compact molecules of lower potential energy. The result of such a change would be an increase in compressive stress in the binder layer as the denatured protein contracts along its linear dimension.
Further work is required in order to quantify the specific effects of water on the albumen proteins. X-ray diffraction and differential scanning calorimetry are methods commonly used to detect protein conformation changes. These techniques could be adapted to help gauge the effects of water on the conformation of albumen proteins molecules. Mechanical studies of cast albumen films would also help establish the rheological behavior of the albumen proteins when exposed to water or water vapor.
The extent of cracking has important implications for the appearance, preservation and treatment of the photograph. Cracked albumen will scatter, rather than transmit a larger proportion of light, causing maximum image densities to loose intensity and depth. A highly cracked albumen layer is more vulnerable to image loss caused by abrasion. Cracks collect dirt, which is particularly disfiguring to highlight image areas. Cracks also expose a far greater surface area of protein and image silver to the detrimental effects of the environment.
The washing of albumen prints is a treatment adapted from both photographic processing and paper conservation. Initial evidence suggests such aqueous treatment of albumen prints is somewhat effective in decreasing overall print yellowing. However, there is also mounting anecdotal and scientific evidence (such as the following illustrations) that indicates washing albumen prints may also increase cracking. Current research, in progress at the Conservation Analytical Laboratory, will address this issue so that a photographic conservator can better balance the benefits of aqueous treatments against the drawbacks.

Fig.1 ALBUMEN PRINT, C.1870, CONTROL, 60X
Untreated albumen print from circa 1870. The white pattern visible in the scanning electron microphotograph is the existing crack network.

Fig.2 ALBUMEN PRINT, C.1870, WASHED, 60X
Another sample from the same circa 1870 albumen print. This sample has been immersed in water for one hour and then dried. Compared to the control sample at the top of the page, the cracks appear longer and wider.

Fig.3 ALBUMEN PRINT, C.1870, CONTROL, 140X
A different albumen print with a pre-existing crack network. Before treatment.

Fig.4 ALBUMEN PRINT, C.1870, WASHED, 140X
A sample from the albumen print shown at the top of the page. Again, the existing crack network has opened after washing.
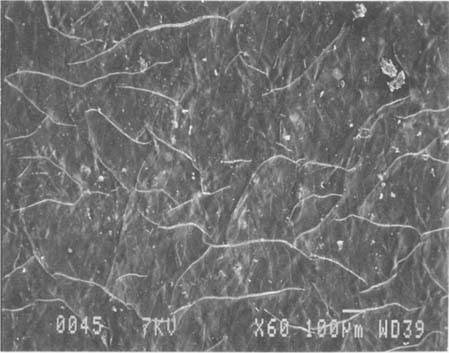
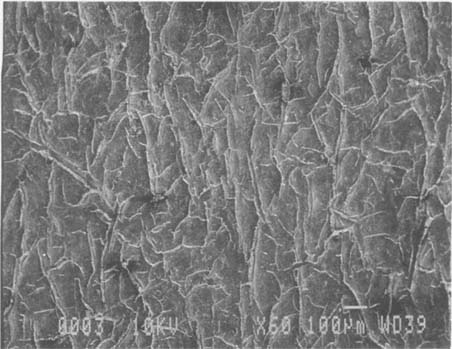
Fig.5 ALBUMEN PRINT, 1989, CONTROL, 60X
This modern albumen print already has a fine network of cracks.

Fig.6 ALBUMEN PRINT, 1989, WASHED, 60X
A sample from the same modern albumen print at the top of the page. After washing, the fine crack network is significantly altered. New cracks are formed. Pre-existing cracks are wider and longer.
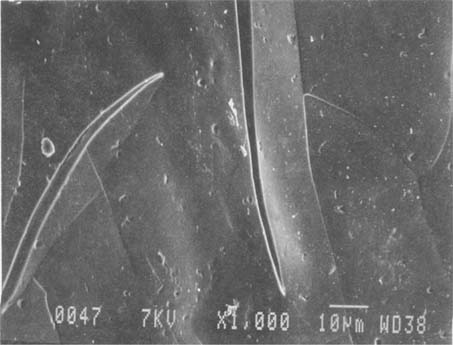
Fig.7 ALBUMEN PRINT, 1989, CONTROL, 1,000X
An example of the fine cracks present in this modern albumen print before treatment. The cracks are narrow and sharp. The surface of the albumen is relatively smooth and in plane.
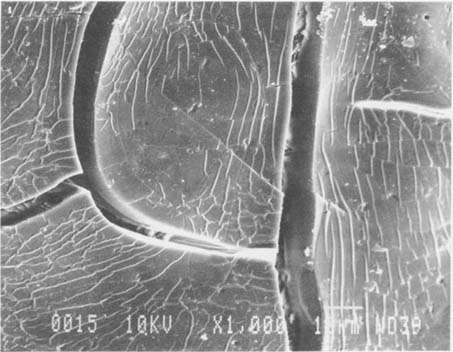
Fig.8 ALBUMEN PRINT, 1989, WASHED, 1,000X
After washing, the cracks have a wider aperture. The surface of the albumen is more disturbed and appears to be cupping. A fine craze pattern has developed which surrounds the major cracks.
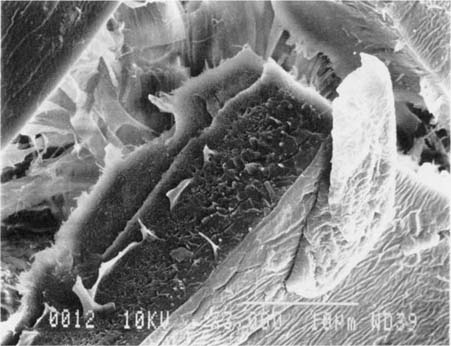
Fig.9 ALBUMEN PRINT, 1989, CONTROL, 3,000X
Observed in near cross-section, the sides of the cracks before treatment are sharp and well defined.
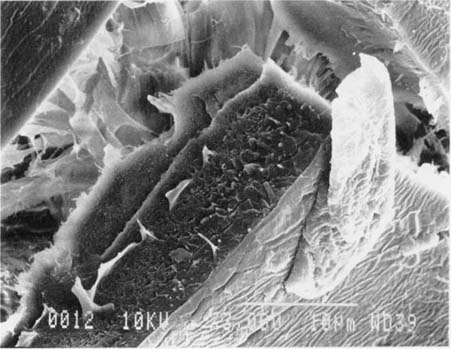
Fig.10 ALBUMEN PRINT, 1989, WASHED, 3,000X
After washing the crack walls exhibit an irregular, “taffy-like” appearance. Note how the crazed surface of the albumen compares to the sample shown at the top of the page.
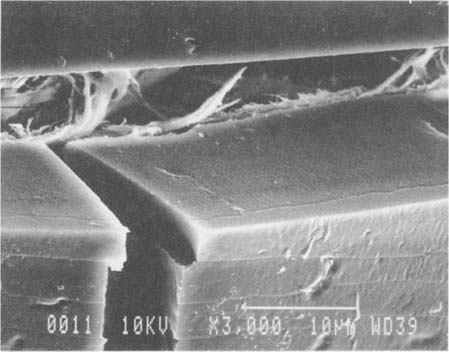
Fig.11 ALBUMEN PRINT, 1989, CONTROL, 3,000X
At close to 10 microns, the thickness of this albumen layer is nearly twice the thickness commonly found in prints from the 19th century. This additional thickness may explain the greater reactivity of the modern albumen prints relative to 19th century examples when exposed to water.

Fig.12 ALBUMEN PRINT, 1989, WASHED, 3,000X
The crazed surface of the albumen has partially flaked off. This type of crazed surface is not present in the samples observed before washing. The crack wall appears fractured and has an irregular surface.
Baker, L., M. Hansen, P. Bhaskara Rao, W. Bryan. 1983. Effects of the presence of water on lysozyme conformation. Biopolymers 22: 1637–1640.
Calhoun, J., A. Leister. 1959. Effect of gelatin layers on the dimensional stability of photographic film. Photographic Science and Engineering 3 (1): 8–17.
Gossett, P., S. Rizvi, R. Baker. 1984. Quantitative analysis of gelation in egg protein systems. Food Technology 38 (5): 67–96.
Karpowicz, A. 1989. In-plane deformations of films of size on paintings in the glass transition region. Studies in Conservation 34: 67–74.
Kuntz, I., W. Kauzmann. 1974. Hydration of proteins and polypeptides. Advances in Protein Chemistry 28: 239–345.
Mecklenburg, M. 1988. Some mechanical and physical properties of gilding gesso. Post-Publication: Gilding Conservation Symposium: In Press.
Powrie, W. 1973. Chemistry of eggs and egg products. In Egg Science and Technology, ed. W. Stadelman and O. Cotterill. Westport, Connecticut: AVI Publishing Co. 61–90.
* Post-Graduate Conservation Fellow, Conservation Analytical Laboratory, Smithsonian Institution.