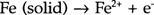
Topics in Photographic Preservation 1993, Volume 5, Article 2 (pp. 8-13)
Photographic negatives and prints in black-and-white in historical collections sometimes show the impression of a fingerprint, generally in a clay-like yellowish color. Rarely is it seen as a blue silvery sheen, known as silver mirror. We have examined the causes of such fingerprints, how they are produced on photographs, and what happens to the image silver in such a case. The long term active ingredients in fingerprints are chloride ions, present as sodium or potassium chloride. These ions are capable of reacting with image silver to form lines of yellow silver salts which mirror the pattern of the fingerprint that caused it.
It has become something of a standard rule in photograph collections that prints and negatives should be handled only by persons wearing cotton or nylon gloves. The purpose of this rule is to avoid fingerprints from appearing on photographs, an occurrence that can be observed on negatives and prints in historical collections. However, the nature of the substance(s) that cause such impressions on photographs, and the changes that they initiate in the photographic image layer have not been clearly defined. The general curatorial literature uses terms such as oily sweats, acidic oils, acidic sweat, and so on, to describe the dangerous chemicals emanating from fingerprints. We have studied the nature of the materials produced by a fingerprint and their effect on black-and-white photographs.
The medical literature concerned with the anatomy and physiology of the human skin that covers our hands describes it as being composed of three layers. Its surface is divided into parallel ridges and valleys which, when brought in contact with an ink pad, form the familiar fingerprint pattern used in the identification of persons. Sweat is produced by sweat glands which are located in the bottom layer of the skin. The sweat migrates to the surface through pore ducts, and exits through pores which are located along the ridges of the skin surface. Our initial speculation was that sweat produced on the inside of a human hand might have a different function — and composition — than the sweat produced elsewhere on the body by strenuous physical exercise, for example, on the forehead. We felt that sweat on our hands may have a lubricating function in view of the continuous use of our hands by touching objects, tools, files, etc. We did not find any evidence to confirm that speculation.
A fresh fingerprint weighs about 10−4g, that is, 1/10 of a milligram. After it has dried, its weight is down to 10−6g. Many analyses of the composition of sweat have been published. While they vary in regard to the exact amount of minor components, they all agree that sodium chloride [NaCl] is the principal component, with some portions of potassium chloride [KCl]. It is present to between 0.7 to 3% of the total weight of sweat. The next most common components are: lactic acid (about 0.3%), and urea (approximately 0.1%). These minor components have a lower ability (about 5%) of causing corrosion in image silver than sodium chloride. The bulk of sweat consists of water. Its pH, when fresh, ranges from 4.5 to 6.7, but rises to above 7.0 into the alkaline region over time.
A fresh fingerprint cannot be easily examined under the microscope, because the liquid film deposited on the surface of a photograph immediately attracts dust particles from its surrounding, which obscure the deposit itself.
In trying to artificially create fingerprints on contemporary photographs, we encountered considerable difficulty. Staff members of our institution placed thumbs and fingers for initially one second, then up to two minutes, on a film which was then exposed to 60°C at 75% relative humidity, for seven days. This generally produced no visible fingerprints on a photograph. We then asked staff members to wear rubber gloves for 30 minutes to support the production of sweat on their hands, with a similarly low success rate. A contemporary processed film with a fresh fingerprint, when exposed to a hydrogen peroxide atmosphere, produced a fingerprint negative: lines of oxidized yellow silver compounds formed where the valleys of the skin were placed. In other words, the sweat deposited along the lines where the ridges of the skin touched the photograph protected the image silver below from reacting with hydrogen peroxide.
We then found that various individuals have different sweat production. A group of individuals, dubbed “rusters” in the medical literature, produce sweat on their hands that has a higher concentration (up to three times and more) of sodium chloride, and therefore is more chemically active. Instrument makers and precision mechanical firms decline to employ such people, as the sweat from their hands may lead to the corrosion of metals. The corrosion of iron in the presence of chloride ions follows a well-understood mechanism.
The generally observed slow reaction of sweat deposited by fingerprints with the contemporary photographic samples prepared in our laboratory may also be explained by the superior hardening of gelatin layers in today's photographic products. It also occurred to us that many of the fingerprints that we see today on older photographs may have been placed there during work in the darkroom by an individual with traces of either developer or a fixing solution on their hands. A simple test demonstrated to us that residual fixing solution on a person's fingers can easily lead to the formation of fingerprints. The yellow lines formed this way likely consist of silver sulfide.
In a wide-ranging series of experiments, artificial sweat solutions were prepared in various concentrations and dropped on areas of different densities in sample photographic prints. These solutions contained from 5 to 30g/L sodium chloride, plus various other ingredients, as suggested by several authors. These ingredients included potassium hydroxide, lactic acid, ammonium carbonate, dipotassium hydrogen phosphate, glycerine, urea, nitrilotriacetic acid, and tri(hydroxymethyl) aminomethane.
The combined results of the application of droplets containing six different artificial sweat solutions to photographic print materials clearly showed that the presence of sodium/potassium chloride is necessary to produce faded spots on silver images.
Droplets containing from 1 to 3 micrograms of sodium chloride produced clearly visible yellow spots on photographic prints ranging from salt prints to albumen prints, Eastman Kodak Studio Proof, Ilford Galerie Paper, and Kodak Commercial Film 4127. The faded spots became visible after 14 days at 60°C and 75% RH.
The mechanism for the reaction of chloride ions with silver may be analogous to that of chloride ions with iron.
If a drop of sodium chloride solution (electrolyte) is placed on a piece of iron and there is exposure to air, the rusting starts after a short time during which a concentration gradient for the dissolved oxygen becomes established. The drop is rich in oxygen at the top, and poor in oxygen at the center. The concentration gradient enables an electrolytic cell action to start within the drop (FIGURE 1). The anode reaction at the centre of the drop is:
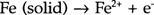
The cathode reaction at the edge of the drop is:
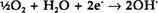
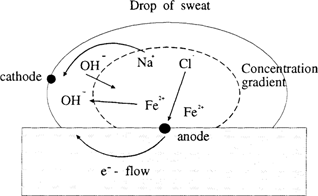
Figure 1: Mechanism of corrosion of iron by a drop of sweat. Anodic attack occurs at the centre of the drop, but the periphery is spared due to the ready access of oxygen to the metal.
The Fe2+ and OH− react to form Fe(OH)2 which is then oxidized by oxygen to form rust, Fe2O3. Sodium chloride is not involved in the process except to carry the electrical current within the drop.
The Nernst equation can be used to show that a similar redox reaction is possible with silver. A fingerprint which contains sodium chloride can be shown to be capable of corroding a silver image. Image silver in photographs, whether developed or printed-out, is physically and chemically quite different from bulk silver. The surface of a developed silver grain contains a high amount of kinks, dislocations, and crystal planes. Therefore the silver surface is more reactive. In addition, the surrounding gelatin is known to influence the corrosion rate of image silver.
We showed that the presence of oxygen, as in the case of the corrosion of iron by chloride ions, is also necessary for a reaction of chloride ions with silver to proceed. Experiments with chloride containing solutions, that produced a reaction on various silver prints in a normal atmosphere, failed to do so when conducted under nitrogen.
The exact nature of the products of the reaction of chloride ions with image silver is not known. In fact, the reaction products of any oxidation reaction of image silver with, for example, hydrogen peroxide have not been identified. In analogy to the above situation, one would expect silver oxides to be formed. In the case of the reaction of chloride ions with silver, there is also the possibility that silver chloride is first formed, which should print out over time under the influence of light to form colloidal silver. In any case, visible discoloration would result.
In summary then, a fresh fingerprint forms a thin liquid film on the surface of a print or negative, which attracts dust particles at once. It consists essentially of an aqueous solution of sodium and potassium chloride, varying in concentration from 0.3 to about 3%, and some twenty additional compound, of which lactic acid and urea are the more common ones. A fresh fingerprint on a contemporary film or print will take some time and favorable conditions to react with image silver. It can be wiped cleanly off the surface of photographs without having caused a lasting effect.
Fingerprints caused by individuals with higher than normal concentrations of sodium chloride in their sweat, or those produced by hands contaminated with either developer or fixing solution, are likely to be the kind encountered most often. It is also probable that photographic materials manufactured fifty years and more ago were more susceptible to the effect of a fingerprint than contemporary materials because of less developed gelatin hardening technology. Residual developer solution will cause fingerprints that are darker than their surrounding, while fixing solution will produce fingerprints of yellow silver sulfide. Fingerprints produced by normal sweat require some time to develop as chloride ions must migrate into the image layer to react with silver particles. The reaction product is either some form of silver oxide, or silver chloride, which will eventually convert to colloidal silver. The visual effect is in all cases a series of bright lines in the pattern of an individual's fingerprint.
The reactions described here illustrate well that only very small amounts of aggressive chemicals are necessary to cause visible discoloration in black-and-white photographic images. The amount of image silver, while varying throughout the technological history of photography and from one type of record to another, is in the range of 50–60μg/cm2. It is finely divided, finely distributed, and has a large surface to volume ratio, making it a reactive material. Degradation reactions of this image silver are fascinating because the amazingly small amount of silver present in a photograph requires only equally minute amounts of reactants to cause striking visual changes in the image that are described as fading or discoloration. Such fading may alter the information content of an image or affect its aesthetic value.
* National Archives of Canada Ottawa, Ontario, Canada