Topics in Photographic Preservation 1993, Volume 5, Article 6 (pp. 52-59)
IPI is engaged in five major areas of research related to the preservation of imaging media:
IPI has proposed the creation of a new ANSI and ISO standard. Its purpose is to provide a standard method to measure the effectiveness of chemical treatments that stabilize silver images. Toner treatments involving gold, sulfur, or selenium have long been known to improve resistance to oxidation, but there has been no objective way to determine if satisfactory protection has been achieved. The new standard contains two tests: the hydrogen peroxide fuming test jointly developed by IPI and Kodak,1 and a simple dichromate bleach procedure. The peroxide fuming test has practical significance because many real-life cases of deterioration can be traced to this class of oxidant. The test is specific to attack by species such as peroxide and atmospheric oxygen.
The dichromate bleach test is a general, non-specific determination of the extent to which the silver image has been converted into a stable, non-oxidizable substance such as gold, silver sulfide, or silver selenide. By measuring the density of the image before and after the dichromate bleach procedure, an estimate can be obtained of the extent to which the silver image has been chemically converted into a resistant compound. (The dichromate bleach removes metallic silver but leaves gold, silver sulfide, or silver selenide unaffected.) The ultimate in stability is achieved when there is no change evident in the image after the peroxide test and when the bleach test demonstrates 100% conversion of silver into a stable compound.
Because very high conversion levels usually result in a change of image color or density, conversion levels used in practice are typically less than 100%. However, unconverted metallic silver is still subject to oxidation by atmospheric oxidants such as ozone or nitrogen oxides. Thus, too low a conversion level would mean that an image is vulnerable to serious information loss due to oxidation. In the new draft proposed standard, the minimum acceptable degree of conversion is 65% (measured as Status A blue transmission or reflection density). This was based on IPI's experience with microfilm, where bleached films having approximately 65% conversion to silver sulfide were used to make acceptable prints and duplicate films.2
SilverLock™, IPI's polysulfide treatment to improve the oxidation resistance of silver images, has been evaluated for use on pictorial films, cinema films, graphic arts films, and photographic papers.3 Originally developed with microfilm in mind, SilverLock has proven to be generally useful with all types of conventionally processed silver photographic media. While optimum treatment times and temperatures do vary for different products, to date IPI has found that polysulfide treatment is successful in conferring oxidation resistance on many different product types and brands.
IPI has continued to develop and improve test methods to evaluate the chemical interactions between storage enclosure materials and photographs. New or improved methods have been developed for specific enclosure components such as inks, adhesives, and plastics, as well as for interactions with specific products such as chromogenic color and diazo microfilm. With the sponsorship of Eastman Kodak Company, a significant new test method has been devised for photograph album pages. All these have been proposed to ANSI-approved committee IT9 for inclusion in ANSI standards. IPI has also proposed that the Photographic Activity Test, which has in fact become a family of related tests, be removed from IT9.2 and made its own separate standard.
One significant alteration has been proposed in the “basic” PAT to remedy a situation where a physical effect could be misinterpreted as evidence of a chemical interaction, causing a harmless enclosure to fail the PAT. Glass, inert plastics such as uncoated polyester, and even some dense, smooth papers tended to cause high levels of fading (relative to filter paper controls) in the colloidal silver fade detector. Such effects were clearly physical and not chemical in nature; for example, a paper product which caused excessive fading when used as a smooth, highly-calendered sheet performed very well when carefully re-pulped into a hand-formed sheet. It was apparent that in certain cases products had failed the PAT which likely would not interact chemically with photographs.
A program of experiments showed that the problem could be corrected by always inserting a layer of filter paper between the enclosure to be tested and the colloidal silver fade detector. This did not suppress the “signal” of harmful chemical interactions—known bad products still failed the test—but it did remove the false signal produced by having a smooth, dense surface in contact with the detector. Modifying the “basic” test in this way brought it into line with the approach used in testing inks and adhesives, where by necessity a layer of filter paper is used between the material and the detector.
The “basic” PAT is designed to explore chemical interactions between enclosures and black-and-white photographs, and the detectors used in the test are specially chosen to highlight possible effects on silver and gelatin. Other types of photographs (for example, chromogenic color prints) may have different reactions to a given enclosure material. Different detectors, incubation conditions, and pass/fail criteria may be needed to evaluate enclosures for such products. IPI has developed a “color” PAT which parallels the basic test in approach, but which uses a 60°C incubation condition and a third detector consisting of the color material of interest. Another such product-specific PAT which IPI has proposed concerns diazo microfilm. In this case, the incubation conditions are the same as in the basic method, but the pass/fail criteria are tailored to the types of dye fading which occur in diazo films.
With support from the National Endowment for the Humanities and the Andrew W. Mellon Foundation, IPI is investigating the effects of four common air pollutants (NO2, H2S, O3, SO2) on various types of films used in micrographic applications. Although the experimental program is only half completed, there are many exciting results so far. Ozone (O3) is a very active oxidizing gas that poses some threat to silver-gelatin emulsions; it is a much greater problem, however, for the organic dyes used in color films and prints. The most sensitive materials appear to be diazo and chromogenic color products. In ozone atmospheres, silver oxidation is rapid at high levels of temperature, humidity, and gas concentration. Lower levels bring a corresponding lengthening of time for equivalent density losses. Ozone affects the gelatin in all products, and the binder may prove to be the most ozone-sensitive element for the black-and-white materials.
In the sulfur dioxide (SO2) to date there is one unusual but significant result. It appears that all of the materials are relatively unaffected by SO2 except for the cyan dye in Eastmancolor negative film 5272. This dye is very sensitive to sulfur dioxide, even in low concentrations at ambient temperatures. Otherwise, SO2 does not seem to be as aggressive toward photographic materials as might have been expected.
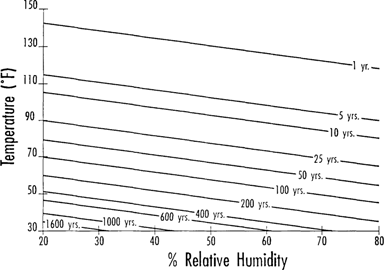
IPI is now in the middle stages of its second large research project on preservation of acetate and nitrate film base. With support from the Division of Preservation and Access of the National Endowment for the Humanities, IPI is investigating several aspects of this important preservation problem. Recently, IPI published Figure 1, which is based on data from the first acetate project in 1988–91.4 This graph summarizes the role of storage conditions in either promoting (or retarding) the onset of deterioration in acetate film base. Figure 1 shows the predicted amount of time required at a variety of temp./RH conditions for fresh triacetate film to attain a free acidity level of 0.5.

Figure 1: Predicted time to reach 0.5 acidity of triacetate film.
The free acidity level of a cellulosic plastic film base is a very good overall indicator of its state of health. All cellulosic plastics (nitrate and acetate alike) are subject to chemical decomposition by acid hydrolysis, in which acetyl or nitro groups become detached, ultimately forming free acetic or nitric acid. Two things happen in the process: the measurable amount of free acidity in the film base increases, and eventually the entire film deteriorates, suffering shrinkage, brittleness, and emulsion damage. This form of deterioration is sometimes given the name “vinegar syndrome” because of the noticeable acetic acid odor which is present in degrading acetate film. The first reliable sign of this degradation process is usually the buildup of acidity in the plastic itself.
ANSI Standard IT 9.1 provides a very sensitive method for measuring the free acidity in films, which involves scraping the emulsion, dissolving 1 gram of film in solvents, and titrating alkali into the solution in the presence of an indicator. The amount of alkali required to neutralize the solution (expressed as mL of 0.1N sodium hydroxide) is a direct measure of film base acidity. Data in Figure 1 was obtained using this method. Newly manufactured triacetate film typically has a free acidity value of 0.05 or less. Films with a free acidity level of 0.5 usually have some vinegar odor but are still usable. Very shrunken and degraded films may have free acidity values of 6 to 12.
Samples of a contemporary triacetate film were pre-conditioned to 20%, 50%, and 80% RH, then wrapped tightly in aluminum foil and sealed in moisture-proof bags. Enough bags were prepared so that samples could be incubated for up to 12 different time periods at each of five different temperatures. The changes in free acidity over time were recorded, and then fitted to a mathematical model known as the Arrhenius relationship. This model assumes that the rate of chemical reactions (for example, the fading of dyes in chromogenic color films) depends in a predictable way upon temperature; if the data from accelerated aging tests at high temperature meet certain mathematical conditions, then extrapolations about the rate of the reactions at room temperature and even under cold storage conditions can be made.
Although actual room-temperature keeping experience has tended to confirm the general accuracy of such predictions (this has been the case with dye fading), care should be taken not to rely too heavily on the specific number of years given in Arrhenius predictions. The best use of Arrhenius data is as a general quantitative estimation; for example, one can have confidence that a prediction of 34 years will be correct to within a decade or two, but the same degree of precision will not hold true when the prediction is for 500 years.
To create Figure 1, estimates of time required for fresh film to attain a free acidity value of 0.5 were calculated using the Arrhenius model. This was done for the range of temperatures from 150°F (65°C) down to 30°F (-1°C). Calculations were performed for samples incubated at each of the three RH's (20%, 50%, and 80% RH) used in the experiment. From this data an interpolated contour plot was created (Figure 1) which relates storage temperature and RH to the estimated amount of time it would take for fresh film to reach the 0.5 free acidity level. The contour lines in Figure 1 are labeled with the time estimates; any point on the lines represents a combination of temperature and humidity conditions which would result in that particular time estimate.
Figure 1 should not be taken to be an estimate of the useful life of acetate film, for several important reasons. First, the 0.5 acidity level represents the onset of vinegar syndrome, not the point at which film is too shrunken or brittle to use. It is a convenient index point to mark a phase of the degradation process where things will begin to happen rapidly from now on. To illustrate this, Figure 2 shows data from an accelerated aging experiment on triacetate film at 122°F (50°C), 80% RH. Free acidity is plotted against aging time in days. The shape of the curve shows that free acidity in the film base increases slowly at first, then increases very rapidly, eventually growing exponentially as a function of time. The 0.5 free acidity point is marked on this curve for easy reference; it occurs at a stage where the free acidity value is about to rise dramatically, but has not yet done so. In a certain sense, it marks the end of a “grace period” where the acidity level has heretofore increased only slowly.
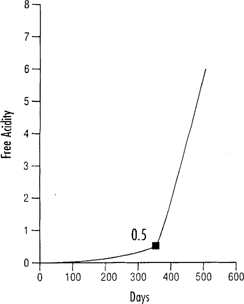
Figure 2: Free acidity of triacetate film incubated at 50°C, 80% RH.
The chemical basis for the shape of the free acidity curve in Figure 2 is the fact that the reactions by which acids are released in cellulose acetate (or nitrate) are autocatalytic. The hydrolysis process which generates free acid is actually catalyzed by acids; in other words, the reaction feeds on itself by continually generating products which speed up the reaction rate. That is why once the vinegar syndrome begins it proceeds relatively rapidly to conclusion. From a preservation viewpoint, it is quite important to prevent film from ever reaching the autocatalytic point.
A second fact to bear in mind about the time estimates in Figure 1 is that the actual behavior of acetate film in real life will be determined not only by the temperature and RH, but also by the packaging and storage circumstances. Acetic acid is a volatile substance that will readily evaporate from film if it can. Allowing the acid to escape slows down the degradation reactions because the acid that evaporates from the film is no longer available to catalyze further deterioration. Experiments show that this factor can have a profound effect on reaction rate.4 One can imagine two extreme cases: one where all the acid is trapped inside the film (this is the worst case, leading to fastest degradation), or the opposite, where all the volatile acid is allowed to evaporate.
Recalling that the data in Figure 1 were obtained from experiments in which a stack of non-interleaved film was tightly wrapped in aluminum foil, Figure 1 represents a situation which is close to the imagined “worst case”. Therefore, in actual practice, film may take longer to reach the 0.5 free acidity level than the estimates given in Figure 1. However, in most real-life storage circumstances there are barriers to the ready escape of volatile acids; film that is tightly compressed in a file drawer or wound upon a reel inside a metal film can is much closer to the worst case of trapped acid than to the ideal of free escape.
One more aspect of Figure 1 to consider is the fact that the time estimates are based on continuous, steady-state temperature and RH conditions for the whole life of the film. In real collections a given film may have experienced a wide variety of storage conditions and packaging circumstances throughout its existence. Its present condition will be determined by the cumulative effects of the varying environmental circumstances it has experienced, and these can be very different from one piece of film to another. In collections having film of varied age and storage history, some materials will still have low acidity levels, while others may be approaching the autocatalytic point. For films which already have elevated free acidity levels, the time estimates given in Figure 1 may be too long.
Given all the ways in which Figure 1 may understate or overstate actual film life, what is its value? Most importantly, Figure 1 provides the best available scientific data on the required storage conditions to ensure that fresh acetate film does not become degraded through the vinegar syndrome. It helps us to understand why one collection experiences heavy losses in only 30 years, while another collection has almost no degradation among 70-year-old materials. Figure 1 is useful for evaluating the relative merits of storage areas for acetate film; sometimes a choice must be made among two or more storage rooms which have different temperature and RH characteristics. Data from Figure 1 can help to decide which one is the most advantageous for storing acetate film. For those considering building new vaults or upgrading their present storage conditions, Figure 1 provides a quantitative statement of the payback in increased film life expectancy that can be expected from investment in improved storage conditions.
With support from the J. Paul Getty Trust, IPI has investigated the dark stability of color microfilm products under a contract with the Commission on Preservation and Access, a preservation advocacy organization headquartered in Washington, DC. Microfilming of brittle books, maps, and manuscripts is an important preservation strategy, but not all such materials can be adequately addressed by black-and-white microfilm.5 The preservation community has raised questions about the stability of color microfilm products and their suitability as surrogates for the original collection material.
IPI's project has examined the dark keeping dye fading behavior, as well as various emulsion and support characteristics of the currently available color microfilm products. These include two families of film: Cibachrome silver dye bleach films and Kodak chromogenic color motion picture films. While the Cibachrome films are specially manufactured for the microfilm application, the chromogenic products are color negative and positive motion picture films which are not specially produced for micrographics.
The project is not yet complete, but several trends are apparent. The dark keeping dye fading properties of the silver dye bleach films are exceptionally good; although these materials do not change enough in accelerated conditions to even allow for Arrhenius predictions to be made, it is reasonable to assume that they would not substantially change in two to three centuries of room temperature 50% RH keeping. The chromogenic films have considerably less inherent dye stability, though with cold storage they too can have such a life span. Data on the physical properties of the emulsion and supports of the two film systems will be reported in the next year.
1. P. Z. Adelstein, James M. Reilly, D. W. Nishimura, and K. M. Cupriks, “Hydrogen Peroxide Test to Evaluate Redox Blemish formation on Processed Microfilm” Journal of Imaging Technology, Vol. 17, No. 3 (June/July 1991), pp. 91–98.
2. James M. Reilly and Kaspars M. Cupriks, Sulfiding Protection for Silver Images. Final Report to the Office of Preservation National Endowment for the Humanities, (March, 1991).
3. Christopher Gmuender, On Black-and-White Paper Image-Stability Enhancement: Effectiveness of Toning Treatments on Silver Gelatin Prints Determined by the Hydrogen Peroxide Fuming Test. MFA Thesis Report, Rochester Institute of Technology, 1992.
4. P. Z. Adelstein, J. M. Reilly, D. W. Nishimura, and C. J. Erbland, “Stability of Cellulose Ester Base Photographic Film” SMPTE Journal (May, 1992), pp. 336–353.
5. Preserving the Illustrated Text. Report of the Joint Task Force on Text and Image (The Commission on Preservation and Access, Washington, DC, 1992.