
Topics in Photographic Preservation 1993, Volume 5, Article 12 (pp. 117-122)
There are three broad types of film-base photographic materials: cellulose nitrate, the cellulose acetates, and polyester. These materials have been used as a support for negatives, positive transparencies, motion pictures, microfilm, and other photographic products. Unfortunately, cellulose nitrate and the cellulose acetates are unstable. Their degradation products can severely harm and even destroy photographic collections, in addition to posing serious health and safety hazards. In particular, institutions should isolate and properly store cellulose nitrate materials because of their extreme flammability, especially when in a deteriorated condition.
The many fires caused by improper storage of cellulose nitrate prompted the advent of the various types of cellulose acetate film-base. Even in their deteriorated state, the cellulose acetates do not have the flammable character of cellulose nitrate and became known as “Safety” film. However, the cellulose acetates do have stability problems. The deterioration of cellulose acetate is auto-catalytic, like that of cellulose nitrate; once deterioration has begun the degradation products induce further deterioration. Because of its increased stability, polyester has replaced the cellulose acetates as a support for some, but not all, film products. A large amount of sheet and roll film remains acetate based because the cellulose acetates can be solvent welded and easily flattened.
The problems associated with both cellulose nitrate and the cellulose acetates have been known for many decades and are well documented. (3,4,5) The instability of film-bases produced before the mid—1950's is particularly problematic. (9) Many of these materials are presently at risk, and their deterioration may place otherwise stable photographic materials at risk as well.
The care and preservation of film-based negatives is divided into four broad categories—Identification; Handling Procedures; Environment & Storage; and Duplication, Rehousing, & Treatment. This essay discusses the last three topics; accompanying charts can be used for identifying individual film—base materials, as well as for surveying large collections. A preservation plan should involve the careful consideration of these four areas. Identification is crucial because storage, duplication, rehousing, and treatment decisions are based on accurate identification.
Film-base materials can be damaged easily, even when in good condition. All three film types, and the gelatin binder on them, can be scratched, abraded, and creased. Oils and dirt from your hands can also damage the support and binder, as well as the final image material.
Once deterioration has begun, film-base materials are even more prone to handling damage. Deteriorated materials can become quite brittle; in this state, repeated removal from a housing can cause considerable harm. Furthermore, deteriorated materials may become sticky and adhere to other materials.
When handling film-base materials, wear clean cotton gloves and work in a clean, well-lit, and well-ventilated area with enough room for processing. Do not allow eating, drinking, or smoking in the processing/examination area. Prolonged exposure to deteriorated negatives can be dangerous, especially when in large collections. Protect yourself by wearing cotton gloves, maintaining good air circulation, using a respirator, not wearing contact lenses, and limiting exposure time. When handling or examining materials establish a system for setting aside or locating damaged materials. These materials should be examined and possibly treated by a conservator.
Maintenance of a proper environment is extremely important to the longevity of all film-based materials. Present recommendations are for a constant environment of 68°F (20°C) between 20% and 30% relative humidity. Current research has found that deterioration is heavily dependent on both temperature and relative humidity. For example, by lowering temperature and relative humidity conditions from 60°F (15°C)/50% RH to 40°F (5°C)/25% RH the rate of deterioration for cellulose triacetate can be slowed by 10 times. (11)
Ideally, each type of film-base material should be stored separately, isolated from other types of film supports. Organizing storage in this way protects other photographic materials from the harmful degradation products of cellulose nitrate and the cellulose acetates. In particular, the nitric acid formed by the degradation of cellulose nitrate can fade silver images, cause gelatin binders to become soft or even tacky, and corrode metal containers and cabinets. This type of material—based organization also makes monitoring the condition of the collection more efficient and effective. Due to the fire hazards associated with cellulose nitrate negatives, it is especially important to isolate any cellulose nitrate materials; in fact, this is required by many insurance policies.
While it is important to separate different types of material if possible, it is also important to segregate deteriorating materials from those in good condition. As mentioned earlier, deteriorating materials produce degradation products that can induce deterioration in other photographic materials.
Three layers of protection are recommended for the storage of film-based photographic materials. Flat materials should be placed in sleeves, sleeves in a box or drawer, and these boxes or drawers on shelves or in a cabinet. Roll materials such as motion picture film and microfilm, should be stored in unsealed containers in cabinets or on shelves. Both flat and roll materials should be kept in a dark area with good air circulation. Ideally, the storage area should have an exhaust system.
All enclosures should pass the Photo Activity Test (PAT) as described in ANSI standard IT9.2-1988.(2) This stringent test evaluates the effect of housing materials on photographic materials. Many manufacturers and suppliers of housing materials now conduct this test on their products. If at all possible, purchase products that have passed the PAT or specify that any housing purchased must pass the PAT.
Sleeves should be made of unbuffered, high alpha cellulose content paper. Ideally this sleeve should be seamless and have no adhesive, although a side seam may be acceptable. The porous nature of paper allows the degradation products to escape from the enclosure, unlike a plastic enclosure which traps those harmful products and accelerates the deterioration of the film material. For the same reason, containers for roll materials should be made of card stock or corrugated board, although other concerns may require use of metal containers. If flat materials are used frequently, a plastic sleeve may be desirable; handling damages are reduced since the material can be seen without removing it.
The specific procedures and extent of duplication, rehousing, & treatment will vary tremendously from collection to collection. However, any approach should rest on a solid foundation of accurate identification of the film-base materials in the collection, a good understanding of the collection's present and future uses, and maintenance of a good environment. Without this kind of foundation considerable effort, time, and money will be wasted. In planning duplication, rehousing, & treatment, factors to be considered are: deterioration levels, size and use of the collection, space available for storage, and financial resources.(6–14)
Recent research indicates that the chemical stability of cellulose nitrate and the cellulose acetates is very similar. Neither cellulose nitrate or cellulose diacetate appear to deteriorate significantly faster than other cellulosic film materials.(11) This research suggests that negative condition and not negative type should be the criterion for duplication.
Decisions concerning duplication, rehousing, and treatment should be considered with a conservator who is familiar with your collection and institution. The deterioration levels outlined by Horvath are especially helpful in determining preservation priorities.(9) These deterioration levels have been listed in the accompanying identification chart. Items from Deterioration Levels 5 and 6 (and possibly 4) should be brought to a conservator's attention. Water damaged materials, and those with mold or signs of insect infestation should also receive a conservator's attention.
In some situations original film-base materials are disposed of following duplication. If disposal is considered appropriate it should only be done after the original and duplicate negatives have been compared, and the duplicate is considered acceptable. Consult with your local fire department before disposing of film base materials, especially cellulose nitrate.
It is vital to continually monitor the condition of the film-base materials in your collection. At this time there is no simple testing procedure for detecting incipient film-base deterioration. The only way to control this problem is to maintain as good an environment as possible and to catch deterioration as soon as it occurs and then isolate the deteriorating materials. This is particularly true of cellulose acetates; their condition can go from an undeteriorated state to a badly deteriorated one in a matter of months, even in fairly good environments. As Horvath concludes in The Acetate Negative Survey, “every institution which contains a substantial quantity of safety film dating from 1925–1955 will find problems with degraded film base somewhere in their collection sooner or later.”(9) Monitoring your collection will allow you to catch the deterioration sooner rather than later. The importance of vigilant monitoring of the collection and its environment cannot be overstated.
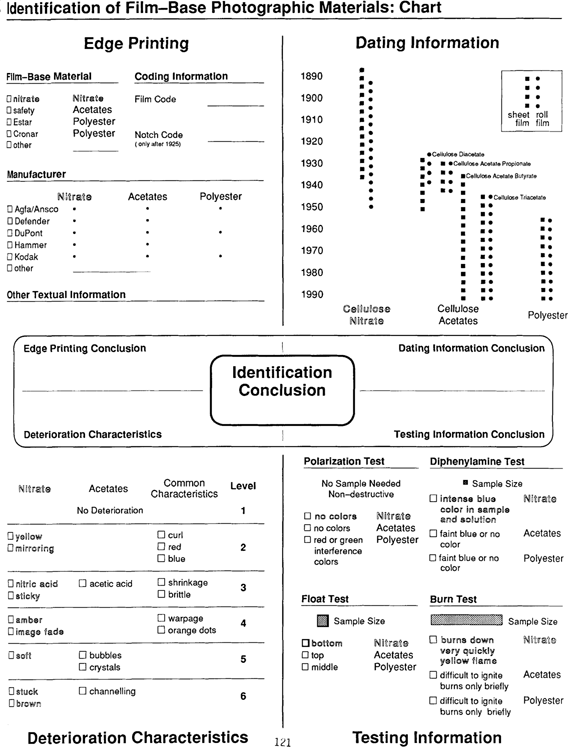
When viewed between cross-polarized filters, polyester and other highly birefringent materials exhibit red and green interference colors like those seen on soap bubbles. Cellulose nitrates and the cellulose acetates do not show these interference colors. The Polarization Test can be performed with the simple viewer illustrated below.
To use the viewer unfold the viewer unfold place a corner of the material in questions over one polarizing filter. Close the viewer and hold the viewer up to a light source. Tilt viewer back-and-forth and side-to-side, red and green interference colors will be most apparent in clear areas. If a material is badly deteriorated, examine it on a light table with one polarizing filter underneath it and one on top of it.

Instructions for Making a Viewer
Trichloroethylene is toxic and a carcinogen! Conduct this test in a well—ventilated area, wear rubber gloves, and use with extreme caution.
The float test may be used to identify film base types due to their differing densities. Cellulose nitrate being the most dense will sink, while cellulose acetate will rise to the top. Polyester should remain in the center of the solution.
Results from this test may be difficult to interpret because deteriorated acetate film may sink to the bottom like nitrate film. Another complicating factor is that the specific gravities for cellulose nitrate and the cellulose acetates fall within a fairly broad range which may cause materials to behave differently. As with the other tests, having a known sample for comparison can be extremely helpful.
Place sample in a test tube of trichloroethylene. Shake test tube so sample is completely immersed. Observe location of sample in the liquid.
Handle this solution with caution. It contains 90% sulfuric acid!
A solution of diphenylamine and sulfuric acid can be used to identify cellulose nitrate. In this solution cellulose nitrate turns a deep blue color. Cellulose acetate and polyester do not produce this color. However, cellulose nitrate is used in very small amounts in the manufacture of cellulose acetate and polyester products. This “subbing layer” does not appear to effect either the longevity or the safety of these materials, but may cause a very faint blue tinge to be seen in the support of the cellulose acetates and polyester.
Place sample on a microscope slide and apply a drop of the prepared solution. After one minute, a cellulose nitrate sample will turn completely blue while the cellulose acetates and polyester will not. In some cases, a large cellulose nitrate sample may exhaust the solution and no blue color will form. Therefore, to confirm a negative test, apply two more drops and wait another minute to confirm that the sample is not cellulose nitrate.
The solution is somewhat sensitive to light. Before testing unknowns, test the efficacy of the solution with a known sample of cellulose nitrate such as DUCO Cement or UHU All—Purpose Clear Adhesive.
Instructions for the preparation of this solution can be found in: Canadian Conservation Institute. (1989). “The diphenylamine spot test for cellulose nitrate in museum objects.” CCI Notes (17/2).
Do not perform in your collection! Cellulose nitrate is extremely difficult to extinguish.
The burn test uses the flammable nature of cellulose nitrate for identification since both the cellulose acetates and polyester are much less flammable. Cellulose nitrate burns quickly and has a characteristic yellow flame. Having known materials for comparison is particularly important for this test.
Hold sample vertically with metal tongs. Be sure to ignite the strip from the top, only cellulose nitrate will burn downwards. For safety, have a large container of water nearby.
Edge Printing may include the name or type of the film—base material in question, especially in the case of the cellulose acetates and polyester. Cellulose nitrate materials rarely contain any edge printing. However, many cellulose acetate and polyester materials do not have edge printing; many never contained edge printing or it may have been trimmed off. Also keep in mind that a copy negative may contain edge printing of the original negative in addition to its own.
Other edge printing information may include: the name of the manufacturer, manufacturing code data, and notch codes. This information may be very useful in identifying materials which have had particular deterioration problems. The Acetate Negative Survey Final Report by Horvath is an invaluable resource for identifying cellulose acetates using this information.
Notch codes can also be used to quickly identify the binder side of the material; when the notch code is in the upper right corner, the binder is facing you.
This chart gives the earliest and latest dates of manufacture of sheet and roll formats of film—base materials in the United States. Manufacturing dates of a specific company may fall within a narrower range than the one shown. For example, Kodak stopped producing cellulose diacetate materials in the early 1940's, while Agfa continued to make them until the mid—1950's.
Like other identifying procedures, assigning a date to a material may be quite subjective. In some cases only a guess from the subject matter of an image is possible; however, in others a date may be found on a housing or in the photograph itself. Even very general information can be quite helpful in identification. For example, cellulose nitrate was the only film material produced before the 1920's, and polyester was not made before the mid—1950's.

Deterioration characteristics of cellulose nitrate and the cellulose acetates, as well as those common to both materials, are listed according to a six level scale used by Horvath. Level 1 indicates no deterioration, Level 6 severe deterioration. If more than one level has been checked off, consider the material to be in the more deteriorated level. These levels can be used to establish Rehousing, Duplication, & Treatment priorities.
The nitric acid produced by the degradation of cellulose nitrate may cause deterioration characteristics of cellulose nitrate (such as image fading and stickiness) to be seen in cellulose acetates and polyester. This can make identification by deterioration characteristics difficult.
While no material is completely stable, polyester is considerably more stable than the cellulose esters and is not included in this chart.
Only inherent deterioration characteristics are included on the chart; it does not include external factors such as poor processing or poor handling.
Tests provide a more exact, but not completely definitive, way of identification. The Polarization test is particularly useful because it is not destructive. The other three tests are destructive; they require that a sample be taken from the film—base material in question. Any destructive tests should be performed only after all other identification procedures have been conducted and identification remains uncertain.
Carefully consider your reasons for conducting a destructive test, it should not be necessary to perform all three destructive tests. For example, the use of the polarization test in combination with the diphenylamine test should make additional destructive tests unnecessary. Many examiners only use destructive tests when attempting to identify a representative sample from a large group.
Do not conduct the destructive tests unless you have been given proper instruction—not only can you cause harm to your collection, more importantly you can harm yourself.

1. Adelstein, P. Z. and J. L. McCrea. (1981). “Stability of processed polyester base photographic films” Journal of Applied Photographic Engineering. 7 (6): 160–167.
2. American National Standards Institute. (1988). American National Standard for Imaging Media-Photographic Processed Films, Plates, and Papers-Filing Enclosures and Storage Containers. No. ANSI IT9.2–1988. This standard is revised periodically; be sure to consult the most recent version.
3. Calhoun, J. M. (1953). “Storage of nitrate amateur still-camera negatives” Journal of the Biological Photographic Association 21 (3, Aug.): 1–13.
4. Carroll, J. F. and J. M. Calhoun. (1955). “Effect of nitrogen oxide gases on processed acetate film” Journal of the SMPTE 64 (Sep.): 501–507.
5. Cummings, J. W., A. C. Hutton, and H. Silfin. (1950). “Spontaneous ignition of decomposing cellulose nitrate film” Journal of the SMPTE 54 (March): 268–274.
6. Eastman Kodak. (1985). Conservation of Photographs. Kodak Publication F–40. Rochester, NY.
7. Eastman Kodak. (1984). Copying and Duplicating in Black–and–White and Color. Kodak Publication M–1. Rochester, NY.
8. Hendriks, K. B. et al. (1991). Fundamentals of Photographic Conservation: A Study Guide. Toronto: Lugus Publications.
9. Horvath, D. G. (1987). The Acetate Negative Survey Final Report. Louisville, KY: Ekstrom Library Photographic Archives, University of Louisville.
10. Puglia, S. T. (1989). “Negative duplication: evaluating the reproduction and preservation needs of collections” Topics in Photographic Conservation 3: 123–134. See also CAN 38: 8–9.
11. Reilly, J. M., P. Z. Adelstein, and D. Nishimura. (1991). Preservation of Safety Film. Rochester, NY: Image Permanence Institute, Rochester Institute of Technology.
12. Sturge, J. M., ed. (1977). Neblette's Handbook of Photography and Reprography—Materials. Processes and Systems, Seventh Edition New York: Van Nostrand Reinhold Co.
13. Weinstein, R. A. and L. Booth. (1977). Collection. Use, and Care of Historical Photographs. Nashville, TN: AASLH.
14. Young, C. (1989). “Nitrate film in public institutions” History News 44 (4, July/August 1989).