Topics in Photographic Preservation 1995, Volume 6, Article 5 (pp. 80-97)
I have recently been surveying the still photograph collection of the B.C. Archives and Records Service. Archivists estimate that the collection includes 250,000 cellulose nitrate negatives which are interfiled with 500,000 safety negatives and 4 million other photographs. As nitrates degrade other materials, I am identifying them so they can be segregated from the rest of the collection, duplicated, and ultimately kept in cold storage.
So far, the bases of 17,608 negatives have been identified, the majority by examination: dating, edge markings, and evidence of deterioration. Ninety-six polyester negatives have been identified by the polarizer test. Five hundred cellulose nitrate and acetate negatives have been tested by the burn1 and diphenylamine2 tests; the trichloroethylene float test was not used due to solvent toxicity. Within this group, 30 negatives gave inconclusive test results. While this is a small number, it held up the survey of 4 collections comprising 34,000 negatives.
I decided to try infrared spectroscopy as a way of identifying problem negatives and refining techniques for the other tests. The tests were conducted at the nearby University of Victoria; the chemistry department offered free training followed by the use of their spectrometer for a minimal fee.
Infrared spectroscopy is a popular instrumental method for identifying organic materials and characterizing their deterioration. With this technique, an infrared beam is transmitted through or reflected from a sample. The sample's component groups will absorb infrared at wavelengths that provide a “fingerprint” of the substance. Fourier transform spectrometers have become popular for conservation applications because they are more sensitive and analyze smaller samples than the traditional dispersive instruments.
Photographic film bases have been tested by the following sampling techniques: potassium bromide pellets,3,4 cast films for transmission,5 analysis of microtomed samples by a microscopic attachment to a FTIR spectrometer6,7 and attenuated total reflectance “ATR” of 35 mm films.8,9 I used both the ATR and the cast film methods.
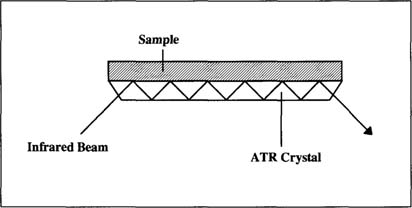
The university chemists recommended attenuated total reflectance. With ATR, a sample is placed in direct contact with a crystal prism “Fig. 1.” The infrared beam reflects inside the crystal; the beam penetrates into the samples before returning back into the crystal.

Figure 1. Attenuated Total Reflectance “ATR”
The university provided a Bruker Fourier Transform Infrared Spectrometer, Model IFS25 with potassium bromide windows. The ATR samples were placed in a Spectra Tech attenuated total reflectance attachment with a 45° zinc selenide crystal prism. Cast film samples were tested both by ATR and by mounting directly in the IR beam. For both sampling methods, spectra were obtained over the region of 4000–700 cm−1. The total number of scans was 32 and the resolution was 8 cm−1.
A library of reference spectra was created from known samples of cellulose nitrate and acetate films in various states of deterioration “Table 1 and 2”. The samples came from deaccessioned photographs and lab supplies. The nitrate motion picture film was a small sample kept for identification purposes; all nitrates had been previously removed from the archives' motion picture collection.
Table 1. Known Cellulose Nitrate Films
Manufacturer |
Provenance |
Date |
Condition1 |
Kodak Canada 35 mm motion picture film |
Sound and moving image collection |
1948 |
Stage 1 (mirror) |
Unknown |
Pocock collection |
c. 1897–1907 |
Stage 1 (mirror, mild yellowing) |
Unknown |
Bishop, from Newcombe collection |
c. 1922–1929 |
Stage 1 (mirror, moderate yellowing) |
Unknown |
George Allen collection negative 1086 |
June 1929 |
Stage 2–3 (adhered to envelope, odor) |
1. The deterioration stages are from James W. Cummings, Alvin C. Hutton, and Howard Silfin, “Spontaneous Ignition of Decomposing Cellulose nitrate Film” Journal of the SMPTE 54 “March 1950”: 271–272.
Table 2. Known Cellulose Acetate Films
Manufacturer |
Provenance |
Date |
Condition2 |
Kodak Sleeve |
lab supplies |
1984 |
Level 1 (no deterioration) |
Kodak Ektachrome |
lab supplies |
1992 and 1993 |
Level 1 |
Ansco (H notch) |
Rogers collection no. 205 |
1951 |
Level 2 (slight curl) |
Dupont (B notch) |
Rogers collection no. 703 c. |
1952 1955 |
Level 4 (warpage, acetic acid) |
Kodak 6142 Super XX (14B notch) |
Ocean Falls collection |
c. 1950 |
Level 2 (mild edge curl) |
Kodak 6146 Super Pan Press (31 notch) |
Ocean Falls collection |
c. 1949–1950 |
Level 2 (moderate edge curl) |
Kodak 6146 Super Pan Press (4F notch) |
Ocean Falls collection |
c. 1949 |
Level 2 (moderate edge curl) |
2. The condition levels and film notch references are from David G. Horvath, “The Acetate Negative Survey: Final Report”, Louisville: University of Louisville, 1987, pp. 68–85.
The initial set of samples was prepared by cutting pieces of film that were equal or larger in size than the ATR crystal “72 mm × 10 mm”. Figures 2 shows a spectrum for the cellulose nitrate motion picture film. The cellulose nitrate can be identified by the peaks 1632 cm−1, 1272 cm−1, and 824 cm−1.10,11,12,13 For comparison, Figures 3 shows the spectrum for cellulose triacetate “35mm Ektachrome”.
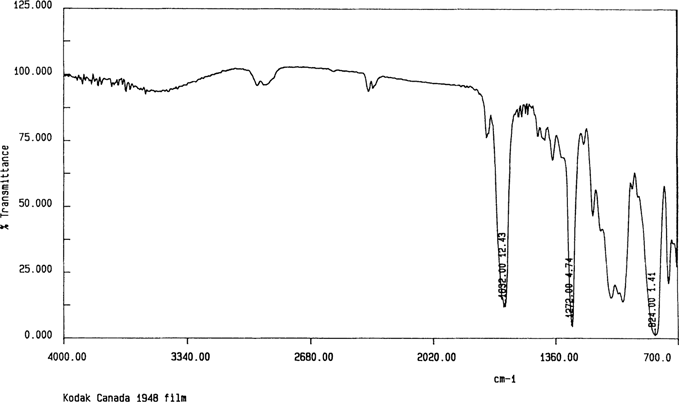
Figure 2
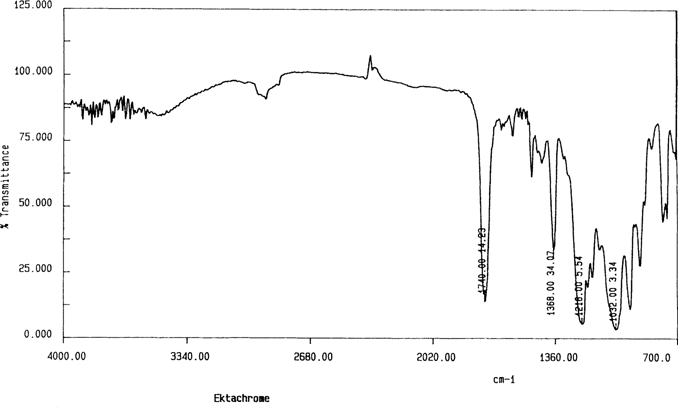
Figure 3
The other samples required removal of the gelatin layers. Figure 4 shows the spectrum for a gelatin cast film and an unprepared cellulose nitrate negative. As the ATR beam penetrated only a few micrometers into the sample, it produced an excellent spectrum for gelatin coatings. The gelatin layers were removed by applying tap water with a swab, scraping away the gelatin with a microspatula, and removing the last residues with swabs of water. The resulting spectra were used as benchmarks for other sample techniques.
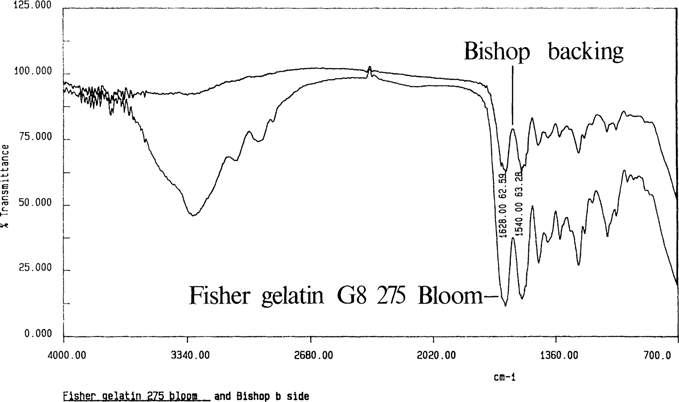
Figure 4
The minimum sample size was determined by trimming down samples of nitrate film until the spectra lost their peaks “Figure 5”. The minimum sample was a piece of film 9 mm × 5mm placed across the crystal.
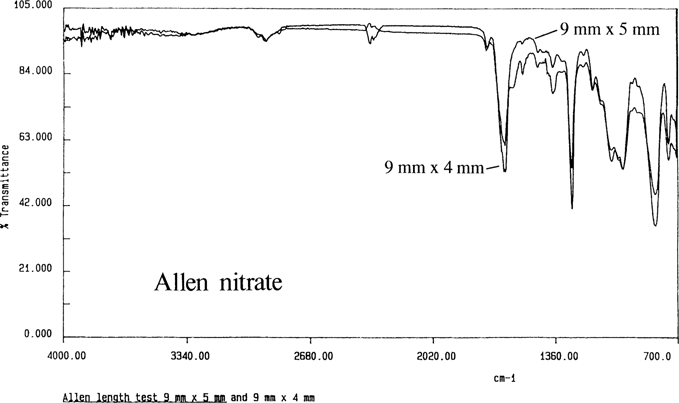
Figure 5
The knowns were next prepared as cast films – on the premise that smaller samples dissolved as a film covered more surface area. The emulsion and backing layers were removed as before. Ethyl acetate was the preferred solvent for all the nitrates, Ansco, and early Kodak films. Cellulose triacetate was soluble in methylene chloride. The degraded Dupont acetate negatives were not soluble in any of the above solvents. Samples were initially prepared by mixing solvent with the sample on a glass microscope slide.14 As the film adhered strongly to the glass, it was separated by immersing the slide in tap water.15 Once the film had floated off, it was removed from the water and dried on polyester sheeting.
Initially the cast films were tested by ATR. I had erratic results for the minimum sample size; generally, results were obtained from samples 15 × 10 mm or more. This can be produced from a triangular piece of photographic film 1/2″ × 1/16″ or larger “13 mm × 3 mm, weighing 2–4 mg depending on the sample”.
Scott Williams of the Canadian Conservation Institute recommended transmission measurements which required smaller samples. Williams recommended mounting samples on a slide mount masked with aluminum foil. Most spectrometers have an IR beam that is 1 cm in diameter; the beam can be masked down to 1 mm which requires a much smaller sample. The film should be in the range of 5 μm to 10 μm in thickness; the thin film adheres by static cling.16
I have been able to take samples as small as 1 mm × 1 mm “the photographic film thickness ranges between .003” and .010″”, producing cast films 5–7 mm in diameter. “The limit for the sample size may be the technique for removing the gelatin layers from the film.” A pinhole 3 mm in diameter produced a spectrum with minimal noise, and still provided enough space around the outside for the film to attach itself.
ATR spectra are similar but not identical to transmission spectra; Figure 6 is the spectrum for the Allen nitrate film by ATR and transmission. The ATR band intensities are stronger at longer wavelengths.17
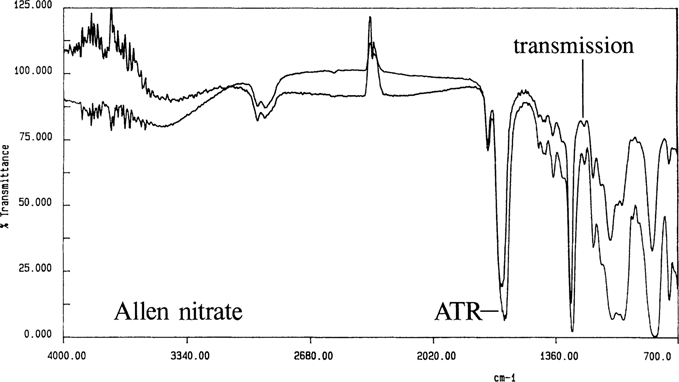
Figure 6. Allen nitrate cast film by transmission and ATR
Through the use of ATR and cast film techniques, acceptable spectra were produced for all of the knowns. Figure 7 show the spectra for the Ansco known by both ATR and transmission. As noted in the literature, the cellulose diacetate could not be differentiated from the mixed cellulose esters. Cast films were produced from all samples except the Dupont acetate which did not dissolve; Figure 8 is the ATR spectrum for the intact film with the gelatin removed.
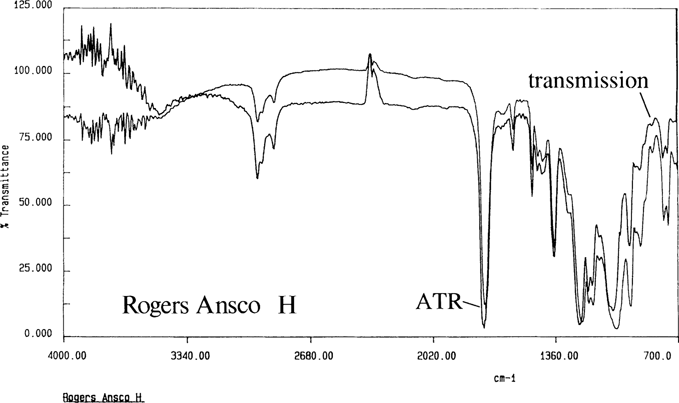
Figure 7. Rogers Ansco H cast film by transmission and ATR
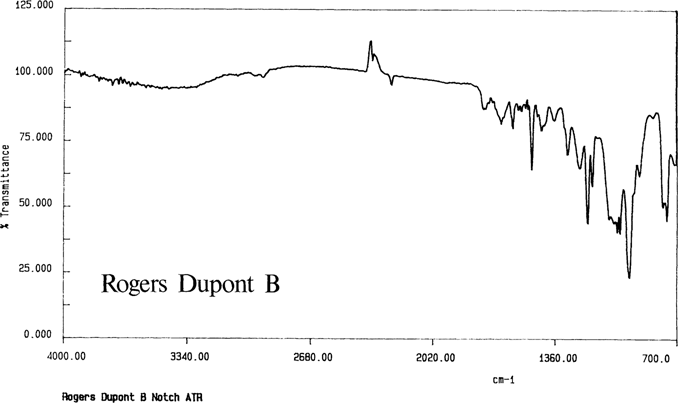
Figure 8
The spectrum for the Kodak safety film shown in Figure 9 indicates another problem with the ATR analysis of intact films; the spectrum is for cellulose nitrate. This negative had been water damaged in proximity to cellulose nitrate negatives. It is presumed that the nitrate spectrum came from either flood contamination or the cellulose nitrate subbing layer. However, the cast film spectrum in Figure 10 indicates cellulose acetate. As ANSI IT 9.1 states that the subbing layer should be removed by scraping,18 another cast film sample was prepared by scraping away the subbing layer with a scalpel. The spectrum was the same as for the cast film with the subbing layer left in place.
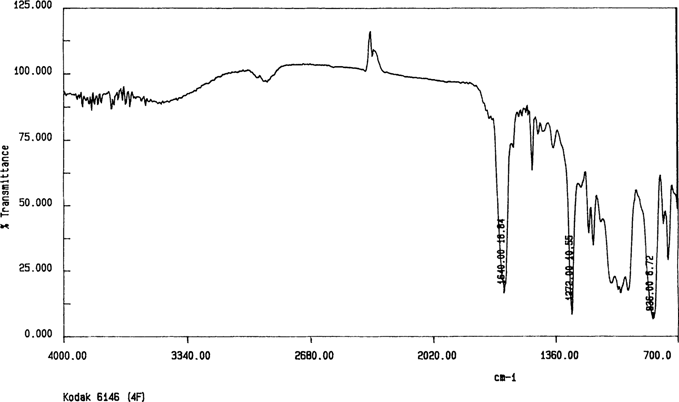
Figure 9. Kodak 6146 “4F” intact film by ATR
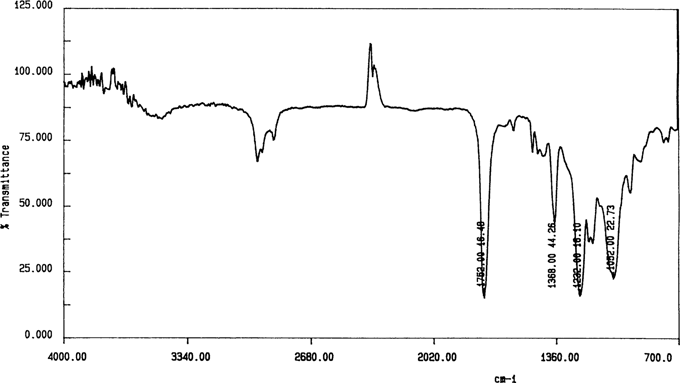
Figure 10. Kodak 6146 “4F” cast film by transmission
Once the sampling techniques were developed, the unknown negatives were identified. Figure 11 shows a spectrum for an unknown negative; the bands are similar to a spectrum for an adjoining Ansco safety negative. Figure 12 shows an ethyl acetate cast film for a cellulose nitrate negative. Figure 13 is a methylene chloride cast film for a 1949 film pack negative; it is similar to the spectrum for Kodak cellulose triacetate. Through the use of FTIR spectroscopy, all of the problem negatives were identified.
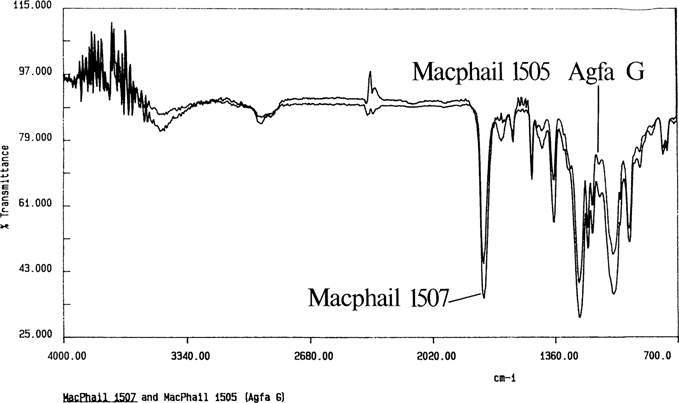
Figure 11
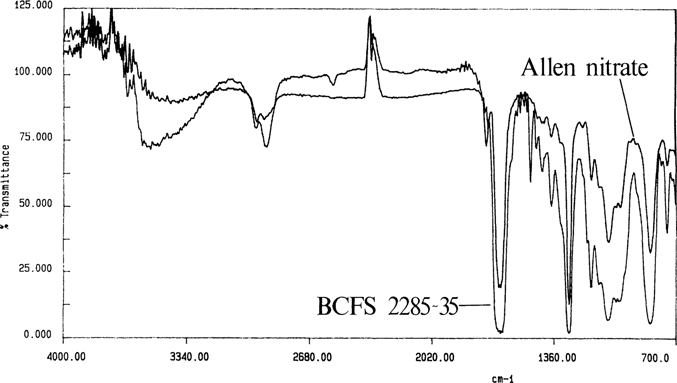
Figure 12. Allen nitrate and unknown negative “B.C. Forest Service 2285–35″
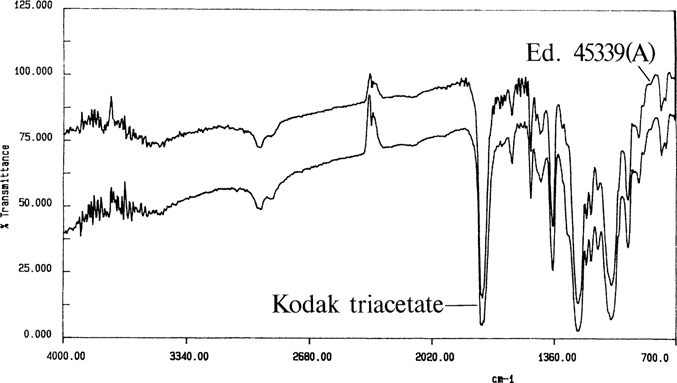
Figure 13. Kodak triacetate and unknown negative “Education 45339A”
The cast film testing method was the preferred identification method. While I was able to use ATR cast films to identify negatives, the transmission films prove to be superior due to their smaller sample size. With both techniques I could prepare samples in my own laboratory with pre-existing materials and equipment, reducing chargeable lab time at the university. After the initial period of refining the sample preparation techniques, testing has become straightforward.
The techniques for the burn and diphenylamine tests were also refined. During sample preparation, I noticed that many samples had thick gelatin layers. Paper micrometer measurements showed that the emulsion and backing layers varied between 12 to 25 micrometers in thickness. This interfered with the initial ATR analyses as the IR beam penetrated only a few micrometers into the negative. For the burn test, it stopped nitrate film samples from burning. In addition, the melting of the acetate film became less obvious. For the diphenylamine test, the thick gelatin layer prevented a fast and strong reaction by the nitrates. There was a narrow difference between this reaction and positive test results for cellulose acetate negatives. Reasons for these results are the cellulose nitrate in the subbing layer, contamination from adjacent nitrates, and, as noted by Fischer and Robb, acetate degradation.19
I could tell the difference by examining cross sections, stained with diphenylamine, under the stereomicroscope. The subbing and gelatin layers turned blue on the acetates. The nitrates' bases would change color, although they would be slow to react when thickly coated with gelatin. If the gelatin “and the subbing” layers were removed, both the burn and diphenylamine tests became more obvious.
Through the use of FTIR spectroscopy, I was able to identify all of the 30 negatives that could not be identified by other means, and the survey could be resumed with refined film testing techniques. At present, film is tested by the burn test, and diphenylamine is used to confirm the results. And IR tests await those negatives that still give inconclusive results.
I would like to acknowledge the generous assistance of the University of Victoria for the use of its infrared spectrometer, and to its staff for training and advice: Karel Hartman, Terri-Anne Hardie, Dr. David Berry, and Dr. Alan Taylor. In addition, Scott Williams “Canadian Conservation Institute” provided a great deal of valuable advice on his spectroscopic techniques. Thanks also go to Charlie Costain and Shelagh Linklater who helped me in the direction of my research. I am grateful to Barry Byers and the BC Archives and Records Service for supporting this work.
1 J. M. Calhoun, “Storage of Nitrate Amateur Still-Camera Film Negatives,” Journal of the Biological Photographic Association 21 “August 1983”: 7.
2 R. Scott Williams, “The Diphenylamine Spot Test for Cellulose Nitrate in Museum Objects,” CCI Note 17/2. “Ottawa: Canadian Conservation Institute, 1994”.
3 American National Standards Institute, Imaging Media “Film” – Silver-Gelatin Type – Specifications for Stability ANSI IT 9.1–1992 “New York: American National Standards Institute, 1992”, pp. 5–6.
4 Perron, Johanne, “The Use of FTIR in the Study of Photographic Materials,” Topics in Photographic Preservation Volume 3. “Washington: AIC Photographic Materials Group, 1989”, p.118.
5 M. Edge, N. S. Allen, M. Hayes and P. N. K. Riley, “Mechanisms of Deterioration in Cellulose Nitrate Base Archival Cinematograph Film,” European Polymer Journal 26 “1990”: 624.
6 N. S. Allen, M. Edge, T. S. Jewitt, and C. V. Horie, “Initiation of the Degradation of Cellulose Triacetate Base Motion Picture Film,” Journal of Photographic Science 38 “1990”: 54–56.
7 Norman S. Allen, Michele Edge, and Terence S. Jewitt, “Degradation and Stabilization of Processed Cellulose Triacetate Base Motion Picture Film,” Journal of Imaging Science and Technology 36 “1992”: 5.
8 A. Tulsi Ram, S. Masaryk-Morris, D. Kopperl, and R. W. Bauer, “Simulated Aging of Processed Cellulose Triacetate Motion Picture Films,” Journal of Imaging Science and Technology 38 “January/February 1992″: 23.
9 W. Van Bronswijk, “Identification of Motion Picture Film Bases by Infrared Spectroscopy,” Journal of the SMPTE 84 “1975”: 476–477.
10 Ibid.
11 Helen Rosenberger and Clarence J. Shoemaker, “Infrared Determination of Nitrocellulose in Mixtures of Cellulose Resins,” Analytical Chemistry 31 “August 1959”: 1315–1317.
12 Julie A. Reilly, “Celluloid Objects: Their Chemistry and Preservation.” Journal of the American Institute of Conservation 30 “1991”: 157.
13 R. E. Kagarise and L. A. Weinberger, Infrared Spectra of Plastics and Resins, NRL Report 4369, PB 111438 “Washington D.C.: Naval Research Laboratory, 1954”: 9.
14 R. Scott Williams, personal communication, 1992.
15 Textile Institute, Identification of Textile Materials “Manchester Textile Institute, 1975”, p.190.
16 R. Scott Williams, personal communication, 1994.
17 J. Haslam, H. A. Willis, and D. C. M. Squirrell, Identification and Analysis of Plastics “London: Iliffe, 1972”, p.30.
18 ANSI, pp. 5–6.
19 Monique C. Fischer and Andrew Robb, “Identification Chart and Guidelines for Care of Filmbase Photographic Materials,” paper presented at the AIC Photographic Materials Group 1993 Winter Meeting, February 28, 1993.
* Conservator, British Columbia Archives and Records Service, Victoria, B.C. Canada