Topics in Photographic Preservation 1995, Volume 6, Article 7 (pp. 106-110)
Centre de Recherches sur la Conservation des Documents
Graphiques, 36, rue Geoffroy-Saint-Hilaire
75005 Paris, France
In France, different non-profit organizations are in charge of photographic collections, negatives and prints, from famous photographers. The goal of these organizations is to ensure conservation and diffusion of their work through exhibition and publications. The best Parisian darkrooms are required to print these images with great care to produce prints that will meet the criteria for long-term conservation. However, over the past ten years, unexpected problems have cropped up. These problems were referred to me, and after analyzing them I was able to suggest possible causes. I would like to take advantage of the opportunity I am given today to present three rarely-described cases that may well turn up in other photographic collections. The presentation will follow the chronological order in which the problem occured.
In 1984 prints were brought to our laboratory. The owners of these pictures had noticed that on the back of these prints, made on baryta paper, were slight yellow spots that were unacceptable on collection prints. We met the printer to discuss details of the procedures and to make sure that they had been carefully carried out; the treatment had clearly been of high quality. Nevertheless, we carried out a preliminary analysis to evaluate the amount of washing. Samples were analyzed according to the ISO 417–1993 standard to evaluate the quantity of residual thiosulfate salts. Since the prints were rather old we used two quantification methods. One of these is quite sensitive “the colorimetric method using methylene blue” but is intended for use on freshly-made prints; the other “the densitometric method with silver nitrate” is less sensitive but is appropriate with pictures that have been processed more than two weeks previously. Neither method showed an excessive concentration of residual thiosulfate. Moreover, there seemed to be no connection between the level of residual thiosulfate and the presence of spots. On the same prints, silver salts were search in the white border to check whether the print was inadequatly fixed. The result of the tests were negative: these prints had been fixed properly.
It was quite by chance that we discovered the nature of these spots. When we placed the print under UV illumination, we were surprised to see a perfect impression left by other prints which must have come into close contact with these photographs. The explanation was now perfectly clear: during processing various prints had come into contact with each other. The optical brightener in the layer of barium sulfate migrated and adhered to the back of the prints with which they were in contact. This transfer of optical brightener was uneven because of the presence of air in the bath, so that the mottled appearance was produced. Paradoxically the yellow colour is not abnormal, since it is the colour of the paper to begin with; the white areas are the abnormal ones. This incident shows that prints should always be carefully separated during processing and that after washing and while awaiting drying they should not be left in contact with each other.
In 1992 another case was referred to us. It involved prints made on Agfa chlorobromide paper. Some of these prints changed colour when they were exposed to light, had been returned to the organizations by the owners. In these cases the image took on an overall warm tone ranging from pinkish red to yellowish brown. This was probably due to photochemical alteration, since the parts protected by the passe-partout had not changed. We were entrusted with two prints from the same series, one of which was deteriorated and the other in good condition.
On the good condition print we took samplings from the image areas. These samples were exposed to xenon lighting at 80% RH and 30°C for five days. After exposure the image had taken on a pink colour. We carried out the experiment again after re-fixing the image with a standard fixing solution; in this case no alteration took place.
The quantitative analysis of residual processing products yielded consistently negative results. We analyzed the images under X ray fluorescence “XRF”. No abnormality was seen, except for the presence of strontium. This could be an impurity in the barium sulfate since it also occurs in store-bought samples of the same kind of paper. Analysis with X ray diffraction “XRD” and in infrared spectroscopy “FTIR-ATR” yielded no useful data. Elementary analysis showed an excess of sodium and chlorine in the deteriorated photographs “Table 1”.
Table 1
elements |
damaged print |
chlorobromide paper: reference |
Na |
220 ppm |
120 ppm |
Cl |
0.61% |
0.12% |
Fragments of these images were also examined by scaning electronic microscope “SEM” using energy dispersive analysis “EDAX”. On all the parts of various photographs that the studied, we detected the presence of chlorine; this chlorine is not present in a reference photographic print “chlorobromide paper”.
As the elementary analysis and the SEM analysis show an abnormal amount of chlorine, this might be present in the form of siver chloride, which, under the effect of light, turns into colloidal silver of a warm yellow or pink hue. This explains why the prints are stable under light after refixing. The source of the component remains to be identified. One might think that the salts were left in the image after poor fixing.
However, if this were the case, the margins of the photo and the lighter areas would also take on some colour. In fact, they do not. Therefore, during washing or storing “in PVC envelopes, poor-quality paper.” the prints must have come into contact with source of chlorine, hydrochloric acid or chloride ions, which reacted with the image to form silver chloride. As regards the processing phase we can put forward a number of hypotheses:
- use of a washing aids containing sodium chloride. This method is called the “seawater wash” and it is occasionally described in the literature as an auxiliary washing procedure.
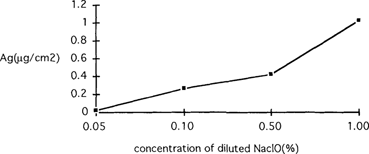
- use of water or hypo eliminator that contain chlorine “such an elimination procedure is mentioned in the literature”. Small quantities of sodium hypochlorite in the rinsing water do lead to the formation of photosensitive silver chloride “detectable through XRD”, and the process is hastened if the bath is acid or acidified by traces of fixing solution. We experimented along these lines with the same type of paper. Photographic prints were immersed in the solution for 30 minutes and then refixed; the amount of silver made soluble by the fixing solution was measured by atomic absorption. Graph A represents the quantity of silver “Ag in micrograms per square centimeters” that turns into silver chloride when the photograph is immersed in a hypochlorite solution at different concentration. These samples, when analyzed by SEM, show the presence of chlorine, silver and also calcium “which is present in the commercial hypochlorite we used”.
Effect of the concentration of diluted 15% NaCIO on the formation of silver chloride in a print after 30 minutes' immersion

In 1994, during the “mois de la photographie” in Paris, there was an exhibition centered on a famous photographer's work. When the prints were hung they were removed from the packing cases in which they had been stored for a few months and it was noticed that several of them were damaged. They were printed on multicontrast classic Agfa baryta paper covered with an 800 g white cardboard passe-partout sold for conservation: the back of the print was protected by visibly low-quality grey carboard. The edges of many images had clearly been oxidized and the discoloration followed the edge of the passe-partout and progressed to the center of the print. Even the high density areas of the image had been affected. When we were consulted about this problem we brought back a sample of each of these materials to the laboratory. A few days later we took another look at the print and we noted that the passe-partout cardboard, which had happened to be in contact with the image, had caused bleaching. Later this area took on a brown colour; this coloration is caused by reduction of the oxidized silver into photolytic silver. But we still needed to find out what oxidizing agent could have caused this deterioration.
We undertook a SEM analysis of the cardboard. It contained chlorine sligthly excess of the levels usual in cardboard. As this analysis was semi-quantitative, we felt the need to determine more precisely the quantity of chlorine present. Elementary analysis through ionic chromatography showed 190 ppm “micrograms per gram” for this cardboard as opposed to 140 ppm for the reference cardboard. This difference in concentration does not seem large enough to explain the board's highly aggressive effect. However, the quantitative analysis does not take into account the initial form of the chlorine, and this can be more active under certain forms than under others. The photographic activity test “ANSI IT 9.16” was applied to this cardboard and to the adhesive used to laminate the 3-ply board. The results are shown in Table 2.
Table 2
|
filter paper |
Glue |
white board |
grey board |
dD colloïdal silver detector |
1.10 |
0.88 |
1.18 |
0.85 |
standard deviation |
0,087 |
0.15 |
0.26 |
0.13 |
% fading |
0% |
-20% |
7.27% |
-22.73% |
dD gelatin staining |
0.08 |
no data* |
0.13 |
0.19 |
standard deviation |
0.01 |
no data* |
0.010 |
0.007 |
staining |
0 |
no data |
0.05 |
0.11 |
mottling |
pass |
fail |
fail |
fail |
Overall performance |
- |
fail |
fail |
fail |
*the filter paper was glued to the detector
These findings struck us as strange at first since on the one hand the cardboard was able to destroy an image in a few days whereas in this case, after 15 days' incubation, only slight discoloration of the colloidal silver detector (7%) is to be observed. However, the very mottled appearance of the colloïdal detector leaves us in no doubt as to the poor quality of the cardboard. The glue, which is a commercial as a dextrine-based glue, showed itself to be quite unsuitable and may be the cause of the rejection of the cardboard by the photographic activity test. Analyses are being carried out at the moment to ascertain the nature of the oxidizing substances.
When these problems appeared, we noticed that photographers and those responsible for collections often underestimate the fragility of silver prints.
But it seems that some, such as chlorobromide prints, are more vulnerable that bromide prints, maybe due to a more tiny silver deposit? Anyway, some of these alterations could probably have been avoided if the images had been toned, however, toning is rarely applied because some chemicals such as selenium, are highly toxic, the procedure increases the cost and also may cause undesirable changes in the hue. These accidents show that cardboards for conservation and exhibition must be selected very carefully indeed. It would probably be a good idea to ask the supplier, whenever board is ordered, to make sure the batches used have been tested and are consistent with standard guidelines.
This work was carried out thanks to the collaboration of Franck Chanteloup, Alain Louvet and Chantal Garnier of the CRCDG, of Bernard Callède of the Research Laboratory of the Monuments Historiques for the SEM analyses and of Myriam Eveno of the Research laboratory of the Musées de France for the XRF analyses.