Topics in Photographic Preservation 1997, Volume 7, Article 7 (pp. 43-54)
This study was initiated at the Image Permanence Institute to investigate the relationship between storage enclosures and the acetate film base degradation known as the “vinegar syndrome.” The so-called “vinegar syndrome” has been studied extensively for the past decade, leading to various preservation recommendations.1 However, the role of film enclosures (e.g., envelopes and cans) and the use of micro-environments to benefit the chemical stability of acetate base film has been only recently evaluated on both sheet and roll films. These two film categories imply different practical approaches in terms of micro-environments and the first experimental results have been reported.2,3 While the storage of film roll facilitates the creation of micro-environments by taking advantage of the individual can, the practical housing system used for sheet films should also take into consideration the nature and design of the primary housing—i.e., envelope or sleeve. This paper addresses the issue of the possible benefit of specific enclosure materials on the stability of acetate base sheet film. The main thrust of this study is the evaluation of the effect of paper alkaline reserve to minimize further degradation of acetate base. Results obtained on the use of plastic sleeves are also reported.
Earlier studies have demonstrated the autocatalytic mechanism of the chemical decay of acetate base, in other words the deterioration occurs at an ever increasing rate by producing its own catalyst mainly acetic acid. This has a practical effect since the storage of deteriorating films with non-deteriorated films represents a common situation in archives which should be avoided. The harmful impact of acetic acid on acetate film is two-fold: (1) as mentioned above acetic acid accelerates the deacetylation of the acetate film base,4,5,6 and (2) acetic acid is also a contaminant for non-deteriorated film which will absorb acetic acid from the environment. Field observations and laboratory experiments have supported this theory.7,8
The climate control option remains the most effective approach of controlling the spontaneous chemical decay of unstable materials such as acetate base.9 However, various technical methods for removal of acid vapors have also been recommended.10 Effective macroclimate measures such as ventilation and acid vapor adsorption (e.g., using activated charcoal filters) benefit the overall environmental quality of storage areas. Recent approaches have addressed only the film's microclimate. The use of acid-scavengers, such as molecular sieves provided by the Eastman Kodak Company11 are intended to act inside the protective film container itself for the preservation of motion-picture films.
For sheet films, the judicious selection of enclosures has also been suggested in order to benefit film stability. This approach may change the role of sheet film enclosures from a “static” one (i.e., protection against physical damage and dust) to an “active” one, involving either the adsorption or neutralization of acidic compounds. Two classes of “active” papers and matte board are currently available to the preservation community: (1) buffered materials, which use various carbonates such as alkaline fillers; and (2) products which combine acid neutralization (provided by calcium carbonate) with pollutant scavenging (by activated charcoal and/or zeolite).12
The impact of enclosures (i.e., nature of material and design) on acetate film stability is part of an IPI investigation (funded by the U. S. National Endowment for the Humanities) to refine guidelines for film preservation strategies. The prime objective of this paper is to document the interaction between acetic acid and enclosure materials for sheet films. Specifically the possible benefits of buffered versus non-buffered paper will be reported.
Natural history collections provided evidence of chemical reactions occurring between acid vapors and calcium carbonate,13,14 and the introduction of alkali fillers in the paper resulted in improved paper stability. Since calcium carbonate has been introduced during the manufacture of buffered paper, it has been assumed that the alkaline reserve of buffered paper might minimize film degradation by neutralizing the acetic acid catalyst produced by the film base. The assessment of such a benefit is the ultimate goal of this study.
The study was based on three types of experiments. In order to explore the interaction of acetic acid on the alkaline reserve of various papers, experiments were carried out with acetic acid adsorption (1) from the atmosphere in vessel experiments, (2) from contacting film in sandwich experiments, and also (3) from contacting film in real-life housing situation. The atmosphere adsorption experiments used glacial acetic acid as the fuming agent in a closed vessel. The two other experiments simulated a situation in which acetic acid was first produced by degradation of cellulose acetate butyrate (CAB) sheet films. The paper test samples were then used either as interleaving material inside sealed bag or as open envelope material. These two procedures resulted in direct contact between the acid source (i.e., degrading sheet film) and the paper.
Four buffered and one non-buffered commercial papers were used for the project. The papers' characteristics are reported in Table I. The initial level of alkaline reserve was determined according to the TAPPI method15 with the exception that potentiometric method instead of the methyl red indicator was used in the titration.2,16
Table I: Characteristics of papers used in the study
Paper code |
Weight (gram/m2) |
alkaline reserve (% CaCO3) |
buffering material |
A |
60 |
3.0–3.5 |
precipitated CaCO3 |
B |
120 |
3.0–3.5 |
precipitated CaCO3 |
C |
90 |
6.5 |
calcite (CaCO3) |
D |
120 |
4.5 |
dolomite (CaMg(CO3)2) |
E |
120 |
none |
none |
The various enclosure papers were exposed to acetic acid vapors inside vessels at room temperature. A series of different acetic acid environments were created by varying the volume of glacial acetic acid injected into a constant volume. The alkaline reserve was used as a measure of the neutralizing reaction.
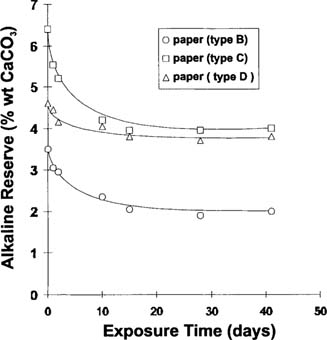
The decrease of the alkaline reserve (expressed in wt % of CaCO3) over a 40 days exposure to an atmosphere of 200–250 ppm of acetic acid is shown in Figure 1. All buffered papers in this study had a limited neutralization effectiveness. Despite the excess of acetic acid used, the alkaline reserve of the papers was never completely neutralized. This observation was confirmed by repeat experimentation over acetic acid liquid during a thirty-five-day fuming period.16

Figure 1: Alkaline reserve for various buffered papers versus exposure time in an acetic acid environment (initial concentration: 200–250 ppm)
It was repeatedly observed that the fuming led ultimately to the simultaneous presence of adsorbed acetic acid in the paper fibers and also non-neutralized alkaline reserve. The presence of both entities can be observed in Figure 2, which shows the pH change with extraction time in water. Samples of buffered (type B) and non-buffered (type E) paper were fumed in the same acetic acid environment (150 ppm) during a seven-day period. At the end of the fuming period, pH measurements were carried out over a six-hour extraction period at room temperature (test samples were not stirred between consecutive determinations). While the pH values remained relatively stable for the non-fumed (type B) buffered paper and fumed (type E) non-buffered paper, a gradual pH increase was observed for the fumed (type B) buffered paper This behavior2 suggests the ongoing neutralization of the dissociated acetic acid by the gradually dissolving calcium carbonate, which is a sparingly water-soluble salt. This interaction is reflected by the pH increase.
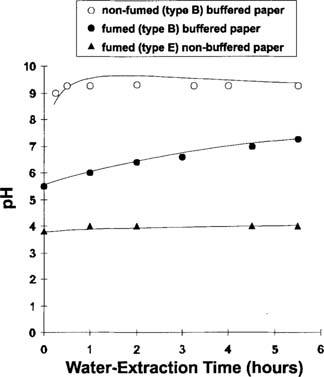
Figure 2: pH versus water-extraction time at room temperature for fumed and non-fumed papers.
It is evident that the fuming of the buffered (type B) paper resulted in a peculiar situation, in which adsorbed acetic acid and residual alkaline reserve coexisted in the paper. It appears that buffered papers behave partly as neutralizing materials (due to the alkali reserve) and partly as acid-adsorbing materials (due to the adsorption by the paper fibers). Over time enclosure papers can be expected to behave as a sort of “acid-reservoir” while there is still “inactive” residual alkaline reserve present.
This behavior of buffered papers is an important practical issue, but it also poses an analytical problem in the evaluation of adsorbed acidity and residual alkaline reserve. Both components can react in aqueous solution although they did not interact during the exposure experiment. A test determination method was refined to evaluate (1) the unreacted acid adsorbed in paper, and (2) the residual paper alkaline reserve.2 The analytical procedure illustrated in Figure 3 was used for this study. Basically, a preliminary step was added to the standard alkaline reserve evaluation. The adsorbed but unreacted free acid was neutralized by titration with sodium hydroxide prior to the subsequent steps of the TAPPI method.15
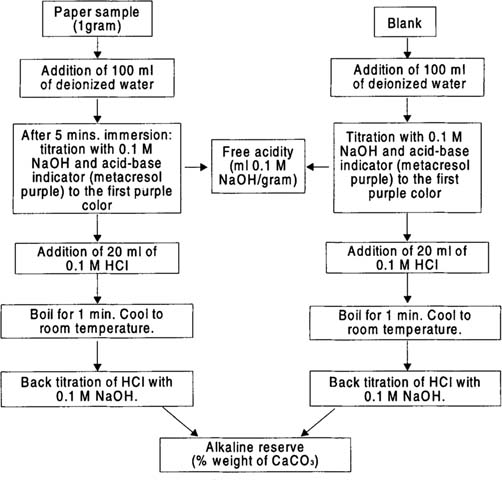
Figure 3: Analytical procedure to evaluate the free acidity and percent alkaline reserve for fumed buffered paper.
The purpose of the experiment was to assess the ability of the enclosure material to control further deterioration of the film in a simulation of a practical storage situation. Cellulose acetate butyrate (CAB) sheet films were first pre-incubated at elevated temperature to initiate the acid hydrolysis of the base, and then reconditioned to 21°C 50% RH prior to incubation in a sandwich format. The pre-degradation acidity level was about 0.2 (ml 0.1M NaOH/gram of film). Sandwich samples were prepared and enclosed in moisture-proof bags for various incubation time periods using the paper samples as interleaving material (see Figure 4).
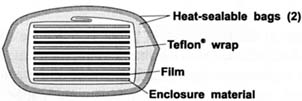
Figure 4: Structure of sandwich samples. Five sheets of cellulose acetate butyrate film interleaved with six sheets of buffered or non-buffered paper, wrapped and sealed in two heat-sealable bags.
The sandwich experiments were incubated at 50°C using both buffered (types B and D) and non-buffered (type E) paper. During a one-year incubation period, the acid buildup within the film was monitored using the water-leaching determination method.17 The free acidity of the contaminated interleaving papers and the residual alkaline reserve of the buffered papers were also measured, following the procedure described in Figure 3.
The acidity change of the CAB sheet films with time is shown in Figure 5. The two buffered papers (types B and D) had a beneficial impact upon the film acidity compared to the film interleaved with the non-buffered paper. The difference observed between the two buffered papers correlated with the different initial alkaline reserve (i.e., 3.0–3.5 wt % of CaCO3 for type B compared to 4.5 wt % for type D). However, it was evident that interleaving with buffered paper did not prevent further degradation at 50°C 50% RH.
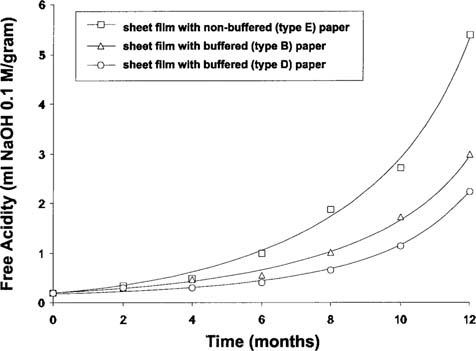
Figure 5: Free acidity of CAB sheet films over incubation time at 50°C, 50% RH in contact with various interleaving papers in sealed bag.
The acid produced by the degradation of the plastic base is distributed in both film and the interleaving paper as a result of acid diffusion. For a complete analysis, the acidity of both the film and paper was determined and their distribution calculated knowing the weights of film and paper. This distribution is illustrated in Figure 6 for non-buffered and buffered interleaving papers.
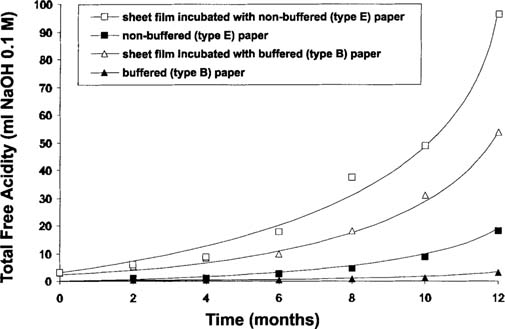
Figure 6: Total free acidity in sheet films and interleaving papers in sample sandwiches.
The acidity increase in the non-buffered paper is evidence that some acid from the degrading sheet films had diffused into the interleaving material. However, the acidity in the non-buffered paper represents only a maximum 19% of the acidity present in the film. This casts considerable doubt on whether interleaving paper can materially reduce degradation of the acetate film base. The beneficial effect of the paper is only a secondary factor.
The acidity level determined in the buffered paper was significantly lower than that in the non-buffered paper. This difference is partly due to the lower acidity generated by the film in the sandwich (see Figure 5), but also because of the action between the acid and the alkaline reserve of the paper. The decrease of the alkaline reserve (see Figure 7) with incubation time indicates a partial consumption of the alkaline reserve of the buffered paper. Separate experiment indicated that the paper itself was stable at these conditions.
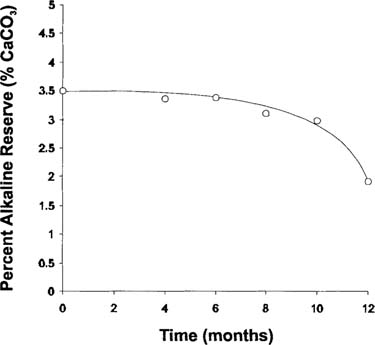
Figure 7: Percent alkaline reserve for buffered (type B) paper, incubated at 50°C in contact with degrading sheet films in sealed bag.
The fact that the acetic acid is partly neutralized by the alkaline reserve indicates that the summation of film and paper acidity does not reflect the total acid produced by the film interleaved with buffered paper. The neutralized acidity also must be taken into account to quantify the merits of buffered or non-buffered paper. The total acidity produced by the CAB sheet films interleaved with buffered paper was calculated to be 30% lower than that generated in the presence of non-buffered interleaving paper after a 12-month incubation at 50°C. This indicates that buffering is of some benefit, but it does not stabilize the acetate base.
The previous results support the hypothesis that calcium carbonate in paper has a neutralizing effect on acetic acid vapors. Consequently, it might be assumed that buffering material would minimize the deacetylation of film by neutralizing at least some of the acetic acid catalyst. However, this conclusion is drawn from data obtained at accelerated conditions. The sealed system and the moderately high temperature both hasten the degradation rate.
Incubation experiments in sealed bags at elevated temperatures may produce results which are difficult to extrapolate to room conditions. This issue was discussed in an earlier paper.2 Three basic mechanisms are involved: (1) the sealed system traps the generated acid vapors, (2) additional moisture (contained in the interleaving paper) is introduced in the sealed bags, and (3) temperature alters the degradation and acid diffusion rates. The former traps the catalyst of the reaction. The second provides an extra-quantity of water to promote hydrolysis of the plastic. A high temperature increases both the deacethylation of the base and the diffusion of acid from the plastic to the surrounding space (e.g., enclosure material). Therefore, investigation conducted at 50°C accurately reflects the behavior at room temperature only if the relative rate of these controlling steps (i.e., acid generation and acid diffusion) are the same. This point has not been established.
Accordingly, experiments should be as close as possible to practical storage situations. Consequently this resulted in two additional studies, first by using open housings, and second by studying the effect at room temperature.
Pre-incubated CAB sheet films housed in individual buffered and non-buffered paper envelopes (filed in vertical position) were incubated at 50°C 50% RH in an open chamber. Table II reports the acidity levels measured during a two-year incubation period. Each measurement was made on a separate sheet of film. As expected open housing lead to a slower rate of acid generation compared to seal bags as illustrated in Figure 7. However, the results do not show any significant merit of one particular type of paper envelope. In both cases, there was an acidity increase with time.
Table II: Acidity of CAB sheet films incubated at 50°C, 50% RH in various open enclosures (acidity levels* are expressed in ml of 0.1M NaOH per gram of film)
Time (months) |
2 |
4 |
6 |
8 |
10 |
12 |
14 |
18 |
25 |
non-buffered paper envelope (type E) |
0.13 |
0.16 |
- |
0.43 |
- |
0.78 |
2.73 |
2.80 |
5.29 |
|
0.14 |
0.16 |
0.23 |
0.41 |
- |
1.05 |
2.59 |
2.20 |
5.24 |
|
0.13 |
0.18 |
0.25 |
0.43 |
- |
- |
2.21 |
2.96 |
- |
buffered paper envelope (type B) |
0.14 |
0.24 |
0.42 |
0.67 |
0.49 |
0.79 |
2.05 |
- |
5.22 |
|
0.18 |
0.24 |
0.47 |
0.69 |
0.67 |
0.82 |
1.36 |
- |
5.02 |
|
0.17 |
0.31 |
0.48 |
0.68 |
0.67 |
0.73 |
2.62 |
- |
- |
fold-lock polypropylene sleeve |
0.25 |
0.25 |
0.37 |
0.51 |
0.70 |
1.42 |
- |
2.84 |
5.05 |
|
0.7 |
0.31 |
0.37 |
0.46 |
0.51 |
1.01 |
- |
2.77 |
5.10 |
|
0.22 |
0.28 |
0.38 |
0.55 |
0.67 |
0.93 |
- |
2.77 |
- |
fold-lock polyester sleeve |
0.25 |
0.25 |
0.32 |
0.55 |
0.73 |
1.08 |
1.28 |
2.54 |
4.70 |
|
0.27 |
0.31 |
0.32 |
0.46 |
0.65 |
1.09 |
2.24 |
3.24 |
- |
|
0.22 |
0.28 |
0.29 |
0.48 |
0.57 |
- |
2.09 |
2.77 |
- |
*acidity measured on separate sheets
The preparation procedure for sandwich samples in sealed bags was the same as described previously. After a 25-month monitoring at 21°C, the acidity results do not display any significant increase or decrease with time (see Table III). Neither the acid-adsorption by the paper fibers nor the acid-neutralization by the alkaline reserve of buffered paper played a dominant role. This experimentation is continuing.
Table III: Acidity of CAB sheet films stored at 21°C 50% RH in sealed bag with three different interleaving papers (acidity levels* are expressed in ml of 0.1M NaOH per gram of film).
Time (months) |
3 |
6 |
10 |
14 |
20 |
25 |
non-buffered paper (type E) |
0.26 |
0.30 |
0.24 |
0.21 |
0.28 |
0.27 |
|
0.25 |
0.29 |
0.29 |
0.29 |
0.26 |
0.26 |
|
0.26 |
0.27 |
0.27 |
0.24 |
0.26 |
- |
buffered paper (type B) |
0.21 |
0.19 |
0.15 |
0.22 |
0.21 |
0.27 |
|
0.16 |
0.24 |
0.16 |
0.25 |
0.27 |
0.28 |
|
0.17 |
0.19 |
0.13 |
0.24 |
0.27 |
- |
buffered paper (type D) |
0.16 |
0.19 |
0.11 |
0.23 |
0.21 |
0.20 |
|
0.25 |
0.22 |
0.12 |
0.17 |
0.23 |
0.20 |
|
0.20 |
0.23 |
0.12 |
0.21 |
0.26 |
0.18 |
*acidity measured on separate sheets
A practical question is whether the alkaline reserve present in buffered paper avoids, or at least reduces, the contamination of non-degraded film by degraded film. To study this effect, alternate CTA film strips (degraded film/non-degraded film) were placed in sealed bags and separated either by buffered paper (type B) or non-buffered paper (type E) envelopes (see Figure 8). The free acidity of the film strips was measured over a 69-day period (see Figure 9). As expected the acidity of the degraded film decreased and that of undegraded film increased. However, neither paper type arrested the infectious behavior of the degraded film. The salient point is that the diffusion occurred through the paper and the alkali reserve present in the buffered paper did not protect the non-degraded film strips. Consequently, it is concluded that paper envelopes do not protect against infectious behavior.
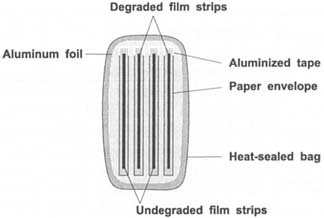
Figure 8: Structure of sandwich samples. Two paper envelopes with degraded film strips and two with non-degraded film strips in alternate positions. Open side of each envelope was taped to force acid diffusion through paper.
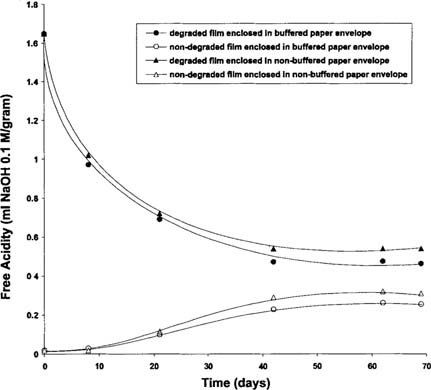
Figure 9: Acidity of degraded and non-degraded CTA film strips stored in alternate positions in buffered or non-buffered paper envelope at room temperature in sealed bag.
The effect of plastic sleeves has been evaluated in parallel with the study of paper envelopes. For this purpose pre-degraded CAB sheet films enclosed in individual fold-lock polypropylene (PP) and polyester (PET) sleeves were incubated at 50°C 50% RH, and also stored at room conditions. These experiments simulated a real-life storage configuration in open housings. The results are reported in Tables II and IV for both plastic sleeves and paper envelopes.
At 50°C there was no advantage of any enclosures. Film housed inside paper envelopes (buffered and non-buffered) or in plastic sleeves degraded at a similar rate (see Table II). At room conditions, the same four configurations lead to a similar observation (see Table IV). A slight acidity decrease was observed but this all reflects the impact of open enclosures. This investigation is continuing.
Table IV: Acidity of CAB sheet films at 20°C 50% RH in various open enclosures (acidity levels* are expressed in ml of 0.1M NaOH per gram of film).
Time (months) |
3 |
6 |
10 |
14 |
20 |
25 |
non-buffered paper envelope (type E) |
0.25 |
0.20 |
0.17 |
0.11 |
0.10 |
0.12 |
|
0.23 |
0.22 |
0.17 |
0.10 |
0.10 |
0.13 |
|
0.245 |
0.19 |
0.15 |
0.09 |
0.13 |
- |
buffered paper envelope (type B) |
0.26 |
0.16 |
0.09 |
0.205 |
0.14 |
0.10 |
|
0.28 |
0.18 |
0.085 |
0.18 |
0.10 |
0.09 |
|
0.27 |
0.165 |
0.08 |
0.10 |
0.145 |
- |
fold-lock polypropylene sleeve |
0.25 |
0.24 |
0.185 |
0.17 |
0.15 |
0.16 |
|
0.27 |
0.19 |
0.165 |
0.195 |
0.15 |
0.14 |
|
0.27 |
0.20 |
0.18 |
0.22 |
0.15 |
- |
fold-lock polyester sleeve |
0.27 |
0.26 |
0.15 |
0.22 |
0.18 |
0.19 |
|
0.29 |
0.23 |
0.205 |
0.195 |
0.175 |
0.11 |
|
0.275 |
0.26 |
0.185 |
0.22 |
0.17 |
- |
*acidity measured on separate sheets
This work was done under a grant from the Office of Preservation, National Endowment for the Humanities. The first part of the study was supported by the Institut Français de Restauration des Oeuvres d'Art, Paris.
1. International Standard ISO 5466: 1992, Photography—Processed safety photographic films—Storage practices.
2. J.-L. Bigourdan, P. Z. Adelstein and J. M. Reilly, “Acetic Acid and Paper Alkaline Reserve: Assessment of a Practical Situation in Film Preservation,” ICOM-CC 11th Triennial Meeting Edinburgh 1–6 September 1996, Preprints, Vol. 2 (1996): 573–579.
3. J.-L. Bigourdan, P. Z. Adelstein and J. M. Reilly, “Use of Micro-environments for the Preservation of Cellulose Triacetate Photographic Film,” IS&T's 50th Annual Conference, May 18–23, 1997, Final Program and Proceedings (1997): 588–596.
4. A. T. Ram and J. L. McCrea, “Stability of Processed Cellulose Ester Photographic Films,” SMPTE Journal 97, no. 6 (1988): 474–483.
5. N. S. Allen, M. Edge, J. H. Appleyard, T. S. Lewitt, and C. V. Horie, “Degradation of Historic Cellulose Triacetate Cinematograph Film: Influence of Various Film Parameters and Prediction of Archival Life,” Journal of Photographic Science 36, no. 6 (1988): 194–198.
6. P. Z. Adelstein, J. M. Reilly, D. W. Nishimura, C. J. Erbland, “Stability of Cellulose Ester Base Photographic Film: Part I—Laboratory Testing Procedures,” SMPTE Journal 101, no. 5 (1992): 336–346.
7. N. S. Allen, M. Edge, T. S. Jewitt, and C. V. Horie, “Initiation of the Degradation of Cellulose Triacetate Base Motion Picture Film,” Journal of Photographic Science 38, no. 2 (1990): 54–59.
8. P. Z. Adelstein, J. M. Reilly, D. W. Nishimura, C. J. Erbland, and J.-L. Bigourdan, “Stability of Cellulose Ester Base Photographic Film: Part V—Recent Findings,” SMPTE Journal 104, no. 7 (1995): 439–447.
9. J. M. Reilly, IPI storage guide for acetate film (Rochester, NY: Image Permanence Institute/Rochester Institute of Technology, 1993).
10. M. Edge, “Factors influencing the breakdown of photographic film: implications for archival storage,” Environnement et conservation de l'écrit, de l'image et du son: Actes des deuxièmes journées internationales d'études de l'ARSAG, Paris, 16–20 mai 1994, (1994): 114–120.
11. A. T. Ram, D. F. Kopperl, R. C. Sehlin, S. Masaryk-Morris, J. L. Vincent, and P. Miller, “The Effects and Prevention of the Vinegar Syndrome,” Journal of Imaging Science and Technology 38, no. 3 (1994): 249–261.
12. W. K. Hollinger Jr, “MicroChamber papers used as a preventive conservation material,” A. Roy and P. Smith Editors, Preventive conservation practice, theory and research: Preprints of the Contributions to the Ottawa Congress, 12–16 September 1994, (London: IIC, 1994): 212–216.
13. N. Agnew, “The Corrosion of Egg Shells by Acetic Vapour,” ICCM Bulletin, Institute for the Conservation of Cultural Material (INC) 7, no. 4 (1981): 3–9.
14. N. H. Tennent and T. Baird, “The Deterioration of Mollusca Collections: Identification of Shell Efflorescence,” Studies in Conservation 30, no. 2 (1985): 73–85.
15. TAPPI Test Method: T 553 pm-92, Alkalinity of paper as calcium carbonate (alkaline reserve of paper).
16. J.-L. Bigourdan, “Behavior of Alkaline Reserve in an Acetic Environment,” ICOM CC—Newsletter of the Photographic Records Group, no. 1 (1994): 14–18.
17. P. Z. Adelstein, J. M. Reilly, D. W. Nishimura, and C. J. Erbland, “Stability of Cellulose Ester Base Photographic Film: Part III—Measurement of Film Degradation,” SMPTE Journal 104, no. 5 (1995): 281–291.