Topics in Photographic Preservation 1997, Volume 7, Article 9 (pp. 66-72)
The deterioration of nitrate film base is an inevitable process in which the decomposition products may act as catalysts for further reactions. Conservators have long been aware of this; the release of the nitro groups, introduced during the original esterification process, followed by the chain scission of the polymer has been widely studied through out the century. However, the behavior of nitrate cinematographic film in archives, even under controlled environmental conditions, is quite unpredictable. The degradation process does not seem to follow the same kinetics among similar types of films and once it starts, the decay proceeds so fast, that there is hardly enough time to duplicate the film. Efforts have been made to find a simple method, which can be applied as a large-scale test, to predict the decomposition of the film before it becomes autocatalytic, thus allowing the duplication of the reel when it is still in good condition. This research evaluates the applicability of direct pH measurement as an indicator of deterioration in nitrate base. To determine the relationship between pH and the degree of decomposition, pH results in 30 film samples, from different time periods, were compared with those obtained by titration, FTIR, viscosity, Tg, and visual inspection. The accuracy of every analytical method, as well as their practical advantages and disadvantages are discussed with respect to their applicability as alternative predictive tests and research techniques
The chemical instability of cellulose nitrate film is still a major problem for most photographic and cinematographic archives. For the latter, the case is even worst than for photographic collections, not only because the stripping conservation treatment is unthinkable for motion picture film, but because decomposition takes place in a very drastic way and duplication processes are complex and expensive.
Although nitrate-base materials are constantly duplicated, the hope of reproducing every single film reel in collections is still remote for most film archives. Therefore, duplication priorities should be established after a careful judgment of the collection's condition is made.
However, conservation assessment can be difficult to accomplish for film archivist; detecting which film-reel would probably decompose sooner and should receive immediate attention, is not always obvious. As it has been observed through scientific research1 and in practice, the behavior of nitrate cinematographic film, even under controlled environmental conditions, is quite unpredictable. The degradation process does not seem to follow the same kinetics among similar types of films, and it can advance, far enough to become autocatalytic, before any symptoms of deterioration are apparent.
After the first symptoms of decomposition are visible the film will go through several stages of decay, in a short period of time, in which duplication turns difficult or impossible. Normally, during the early stages the image will start fading and the gelatin will soften. Then, as discoloration and softening of the film support proceeds, different parts of the film reel may adhere together until it becomes a single mass. Finally, this semi-cristaline mass will turn in to a brown pungent powder.
The chemical reactions which are responsible of this sudden decomposition have been described as the release of the nitro groups introduced during the original esterification process, followed by the chain scission of the polymer; reactions take place via acid hydrolysis or thermal oxidation.* (see bibliography)
Although the autocatalytic nature of nitrate decomposition is not exclusive of motion picture film, but common to any nitrate-based film format, the process has a remarkable impact in the context of cinematographic archives; considering the amount of film in every cane and the way it is stored, decomposition products will easily accumulate in sufficient amount to turn the process autocatalytic.
Because the deterioration process cannot be detected visually during its incubation period, conservators require a test: an indication of the imminent decomposition of a certain reel.
Efforts have been made to find a simple method, which can be applied as a large-scale test, to predict the decomposition of the film before it becomes autocatalytic, thus allowing the duplication of the reel when it is still in good condition.
This not a new idea, several methods of this kind were developed since the 50's2 and particularly one of them, the Alizarin Red test, is still used in some film archives.3 Owing to the formation of nitrous and nitric acids in the film as a result of it's decomposition (in the presence of moisture), most tests have been designed based on the sensitivity of organic dyes to pH changes. In the Alizarin Red test, for example, as dyed paper strips are exposed to acid vapors released by the film samples, fading will occur at different rates depending on the grade of decomposition of every reel.
Following this idea, it was my intention to investigate the real possibilities and usefulness of applying a predictive test based on pH value; and in this way, help curators in deciding weather it is worthy or not to introduce an aging test as part of their daily work.
Although the vinegar syndrome is at the present the main concern among film conservators, for many archives in Mexico, which do not have environmental control systems in their vaults, the preservation of nitrate collections is still the most urgent task, although a real challenge.
This research project was intended to be useful for any film archive in the country, but specially for the National University Film Archive (Filmoteca de la UNAM) which is the largest historic film archive in Mexico. Because their vaults are better than many others in terms of fire prevention, UNAM film archive have been acquiring several nitrate collections and expect to receive more in the future. Owing to the large number of titles which might require duplication and previous storage conditions are usually unknown, they need to survey recent and old acquisitions in order to establish priorities and design long term preservation programs.
Following research methodologies and survey strategies developed by several conservation scientists, it was my intention to propose an alternative testing method applicable to the Mexican film archives.
Based upon the different kind of transformations which cellulose nitrate may suffer during aging, several analytical techniques have been applied to study it's decomposition process. Desterification and chain scission reactions, although related in the deterioration process, may produce different type of physical and chemical changes; accordingly, they should be considered separately, as parallel or complementary reactions when selecting the analytical methods and designing an aging test‥ Following publications by the Image Permanence Institute4, and their experience of acidity as the best indicator of film deterioration (in this case desesterification), pH appeared as the most feasible test to evaluate.
To determine the relationship between pH and the degree of decomposition of the film and evaluate it's role as indicator, results in 30 film samples, from different time periods, were compared with those obtained by other methods listed below. The accuracy of every analytical method, as well as their practical advantages and disadvantages were studied with respect to their applicability as alternative predictive tests and research techniques‥
Titration of soluble acidity: Extraction of the nitric acid associated with the desterification process.
Relative viscosity: Because the viscosity of a polymer is determined, among other factors, by molecular weight, the decrease in molecular weight as a result of acid hydrolysis or thermal oxidation can be used as a parameter of deterioration.
Termogravimetric analysis (Tg): Thermal stability of the film is related to it's level of decomposition by the fact that as nitrate decay's it become more vulnerable to thermal oxidation.
FTIR spectroscopy (transmission): Spectroscopic analysis have been used to identify modifications in the structure of the polymer as a result of the desterification process.5 Basically, a decrease in the intensity of the nitro absorption bands should be detected as NO2 groups are released. Because this group will be replaced by C=O (carbonyl) and in some cases O-H (hydroxyl), an increase in the absorption bands corresponding to these two groups should be evident by IR spectroscopy.
Visual inspection of film samples Thirty film samples, representative of different nitrate collections, were selected for this research. For most cases, to avoid more damage to unique film titles, only one picture frame was obtained to perform all testing procedures.
In order to correlate every physical aspect in the film stock with the results and the behavior of the samples during testing a data sheet was filled for every sample containing the following information: year in which film stock was produced, the type of film stock (positive, negative), type of sound (if any), manufacturer, presence of tinting or toning, type of perforation, type of container, amount of film in the container, storage conditions (present and past), part of the reel where the sample was taken, visual evidence of deterioration, (among others).
Advantages - Even though FTIR spectroscopy could not be applied as a quantitative analysis, it is a useful research tool to identify the decomposition products of cellulose nitrate and study it's mechanism of deterioration. The release of nitro groups and the subsequent formation of carbonyl structures was evident in several aging tests carried out in new cellulose nitrate resin. Although the composition of this lacquer varies in degree of nitration and polymerization from the original material used as film support, monitoring it's aging behavior by FTIR for an incubation period of nine months, was useful to understand the general decomposition mechanism of this kind of polymers.
Disadvantages - FTIR spectroscopy showed not to be sensible to denitration during induction period. The decrease in the content of nitro groups and their substitution with carbonyl were detected only in films in last stage of deterioration. So, different levels of chemical change among several film samples could not be measured by this technique. Although a slight tendency in the increase of C=O was observed in more deteriorated film samples, this was not clear and constant in all cases. It might be possible that nitro groups get trapped in the polymeric structure, owing to the tight atmosphere which surrounds the film during storage, so no decrease in the content of this group can be observed through IR spectra.
An interesting observation about FTIR is it's sensitivity to film age; several differences between the spectra of early film samples (produced before 20's) and the later ones (from the sound period) were observed. The first ones showed a poor resolution with respect to the later ones, of higher definition. This can be interpreted as differences in their formulation, finding more impurities in early film stock from the silent period than in later production.
Advantages - Tg is an accurate and is less time consuming method as film samples do not require any special preparation (the presence of emulsions does not modify the results) and only a minimum amount of film is needed. Thermal stability can be used as a parameter to compare the deterioration level of different films, specially if they are contemporaneous.
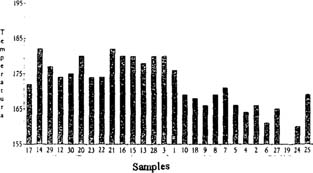
Disadvantages - Even though Tg analysis demonstrated the relationship between the level of deterioration of the films and their thermal stability (resistance to oxidation), results were influenced by their age: that is, the time period in which every film stock was produced. A difference between the behavior of early film samples and late film samples was observed. The first ones will decompose at a lower temperature but in a less drastic way, and leaving a higher amount of residues after the test is completed. This can be interpreted as a lower degree of nitration and polymerization, due to aging or simply because the formulation was different. Fig 1

Fig. 1: Temperature of decomposition plotted vs. the age of the film samples. Starting from the left with the most recent film stock.
Advantages - Viscosity analysis showed a remarkable sensibility and accuracy to detect the progressive decrease in molecular weigh of the film. Viscosimetry should be complementary to acidity tests which are subject to failure.
Disadvantages - As it is the case of Tg analysis, viscosity results are also influenced by the age or date of manufacture of the film; a certain tendency of early film samples to give smaller viscosity numbers was observed. Besides, the test is very time consuming.
Advantages - pH value certainly relates to deterioration. Although it's level of accuracy is not as high as titration, it clearly showed a correspondence with changes in the properties measured by other techniques. Fig 2
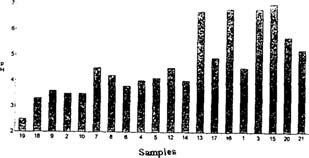
Fig. 2: pH values vs. level of deterioration. Samples are placed (from left to right) in a decreasing order of deterioration.
Only a small amount of sample is required to obtain a pH value different enough, from one sample to another, to judge the condition of a film in relation to the rest. The test is easy to perform making it useful as an indicator of the level of decomposition within a large collection
Advantages - Acidity was the most sensible and reliable indicator of film deterioration. Except from the cases where emulsion layer dissolved, acidity showed a constant relation when plotted against the level of deterioration detected by other methods and visually. Because the amount of acid cannot be exactly the same in two different films, titration can give very accurate results even with small samples. Fig. 3
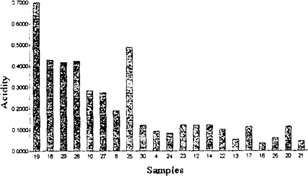
Fig. 3: Soluble acidity vs. degree of decomposition. Samples are placed (from left to right) in a decreasing order according their deterioration.
Disadvantages - Acidity values are more vulnerable or ephemeral than other properties; results can be influenced by the kind of storage or environmental conditions. in which film sample was kept.
The influence of possible contaminants which may dissolve in the water during measurements can modify real acidity values of the films. Specially due to aminoacids from the gelatin binder when acid hydrolysis has turned it soluble.
Although emulsion layer can be considered as a source of contamination for either pH test or titration, it is also the main source of information since most acid is trapped in this layer. It was found, after removing it from several samples, that most of the acid which should be present was gone. So, gelatin layers should not be removed but the influence of amino acids and carboxylic acids should be kept in mind when interpreting pH or titration results.
In the case of Titration, because of the great difference in the amount of acid from sample to sample, the concentration of the neutralizing solution must be determined every time; finding the right concentration of sodium hydroxide solution for different film samples can be very time consuming.
- There was no single test completely reliable to determine the stage of decomposition of the films, as results can vary depending on the property which every analytical method is able to measure. Techniques do not only differ in their level of accuracy but in the type of physical or chemical changes which they can detect. Never the less, results were constant for films which are on the extremes: either in very good or in very poor condition, indicating the sensibility of most methods.
- Considering the simplicity of the pH test and the low amount of sample required to obtain an individual value for a film, differentiated enough from the rest, it's level of accuracy was acceptable; if carried out in a systematic and controlled way and keeping in mind possible distortions of pH values due to contaminants trapped in the emulsion layer.
- pH values showed a drastic droop from samples which were in good conditions (according to all other tests) to samples in doubtful conditions and of course with those which were obviously degraded. The lack of intermediate values corroborates the rapid increase of acidity in the film due to the autocatalytic nature of decomposition.
- In every case, but mainly for acidity tests, sampling method is critical. Acidity values can mask real deterioration stage when, for any reason, the film was not keep in a tight container. So it is advisable to avoid films which have remained out of their container or in any extraordinary condition; if possible, samples should be obtained from the center, close to the spool.
- pH test as well as titration should be complemented with viscosity measurements in order to corroborate the chain scission.
- Observations in every sample confirmed the idea that the deterioration of nitrate film is not related to any particular characteristic of the film stock
Because nitrate film stock was not longer produced after 1951, absolute amount of physical or chemical changes due to aging cannot be measured. Film samples can only be compared with those reels which are in better conditions, so results should be used as a relative scale to survey or evaluate the collection.
Although the preservation of Motion Picture Film is considered as a distinct field, separate from still photography preservation, similarities in the materials we both deal with, should allow photo-conservators to contribute to the preservation of film collections. Specially in a country like Mexico, where the development of both fields is still very limited.
Film samples were obtained from the Filmoteca de la UNAM, Cineteca Nacional, and the Archivo Histórico Cinematográfico de la Fundación Carmen Toscano.
1. Reilly, J., IPI Storage Guide for Acetate Film. Image Permanence Institute, Rochester, NY 1993.
2. Calhoun, J.M., “Storage of Nitrate Amateur Still-Camera Film Negatives” J. Biological Photographic Association Vol. 21, No. 3 1953
3. Brown, H., Basic Film Handling, FIAF Preservation Commission, 1985
4. Reilly, J., Adelstein, P., Nishimura, D., Final Report to the Office of Preservation, National Endowment for the Humanities. Preservation of Safety Film. Grant # PS-20159-88. Image Permanence Institute, RIT, 1991.
5. Edge, M., Allen, N.S., Hayes, M., Riley, P.N.K., Horie, C.V., “Mechanisms of Deterioration in Cellulose Nitrate Base Archival Cinematographic Film”. Eur. Polym. J. Vol. 26, No. 6, 623–630, 1990.
- Adelstein, P., “From Metal to Polyester” History of Picture-Taking Supports. S.P.S.E., The Society for Imaging Science and Technology, Northeastern University Press 1987.
- Adelstein, P.Z., Reilly, J.M., Nishimura, D.W., Erbland, C.J., “Stability of Cellulose Ester Base Photographic Film”. SMPTE Journal, May 1992.
- Edge, M., Allen, N.S., Jewitt, T.S., Horie, V.C., “The Long-Term Stability of Cellulose Ester Coating”, Polymer Degradation and Stability, No. 26, 221–229, 1989.
- Louvet, A., “Study of the Degradation of Cellulose Nitrate photographic Base” ICOM Committee for Conservation. Photo. Newsletter Bulletin, No. 1, 23–24, 1994.
- Reilly, Julie A., “Celluloid Objects: Their Chemistry and Preservation” AIC Journal. Vol. 30, No. 2 145–162, 1991.
- Selwitz, C., “Cellulose Nitrate in Conservation”. Research in Conservation No. 2, The Getty Conservation Institute, 1988.
- Tulsi Ram, A., McCrea, J. L., “Stability of Processed Ester Photographic Films”. Eastman Kodak Company, 14650, 1987.
* Fulbright Fellow-Image Permanence Institute -RIT/George Eastman House/Escuela Nacional de Conservación, Restauración y Museografia. MEXICO. *This research was presented at the National School for Conservation, Restoration and Museography, Mexico City as a thesis paper to obtain the degree in Conservation. It was an interdisciplinary project between the School for Conservation and the Faculty of Chemistry and the Institute for Materials Research from the National University of Mexico. Thesis Advisor: Ernestina Cervera