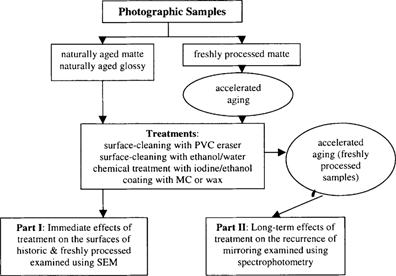
Topics in Photographic Preservation 1999, Volume 8, Article 6 (pp. 31-43)
In this study, a selection of treatments for silver mirroring were examined with regard to their immediate physical effects on the gelatin surfaces of naturally aged and freshly processed silver gelatin photographs. The treatments included the use of poly(vinyl chloride) erasers, the application of ethanol/water or iodine/ethanol solutions, and coating with methyl cellulose or wax. The surfaces of both the naturally aged and freshly processed photographs were examined visually and with a scanning electron microscope. The degree of silver mirroring recurrence was studied using UV-vis spectrophotometry on the freshly processed, treated samples after artificial aging. The results suggest that certain treatments may have detrimental effects on the surfaces of silver gelatin photographs. The iodine/ethanol treatment provided a stabilizing effect on the silver image particles of the samples.
Silver gelatin photographs can suffer from a form of image deterioration commonly referred to as silver mirroring. In some cases, the “mirroring” may be considered disfiguring as it generally forms unevenly and, if extensive, can obscure image detail.
The phenomenon of silver mirroring has been studied over the years generating much speculation as to its formation and composition. A common theory on the formation of silver mirroring which emerges from different studies suggests this form of deterioration is a product of an oxidative-reductive mechanism which is promoted by prolonged or excessive exposure to a combination of moisture, oxidants, and the appropriate reducing agents in the environment. The mirroring is formed more readily in the presence of moisture and is accelerated at higher temperatures. In the process, the silver ions formed by the oxidization of the silver image particles are able to migrate within the emulsion and to the surface of the print where they are subsequently reduced to a visible metallic form. Ulla Bøgvad Nielson (1993) provided a more in-depth explanation for the phenomenon based on her studies and summarized the models for silver mirroring formation proposed by Edith Weyde and Klaus Hendriks.
The treatment of silver mirroring carries with it some controversy as well, in particular those methods that remove the silver mirroring. Some practitioners argue that the mirroring should not be removed, as it is part of the original image material, albeit relocated within the emulsion and converted to a different form. It is considered by some a natural form of image aging not unlike a patina.
A number of methods, however, have been used experimentally and in conservation practice to treat silver mirroring. While some of these treatment methods are reasonably well established, little research has been carried out on their immediate and long-term effects on the photographic image and binder. Ulla Bøgvad Nielson (1993) studied the treatment of silver mirroring with regard to its long-term effect on image stability. Nielson examined the image stability of naturally aged, developed-out silver gelatin photographs that had been treated using a selection of methods that included an iodine/ethanol solution and poly(vinyl chloride) (PVC) erasers. After subjecting the samples to accelerated aging using the ANSI IT-9–1992 peroxide test with added Kodak brown sulfur toner, she concluded that the treatment with the iodine/ethanol solution followed by fixation and washing successfully removed the silver mirroring and appeared to have a stabilizing affect on the remaining silver image (Nielson 1993).
Two related studies addressed the immediate effects of treatment on the photographic gelatin surface. Although these studies were based on techniques for surface—cleaning photographs with the intent of only removing superficial “dirt” and not silver mirroring, some of the treatments discussed have also been used in practice to remove silver mirroring. Brenda Bernier (1997) examined two types of PVC erasers and their effects on photographic surfaces including naturally aged, silver gelatin developed-out photographs. She found no visible signs of surface abrasion when the samples were examined using a stereomicroscope (Bernier 1997). She suggested, however, that the use of scanning electron microscopy (SEM) for the study of surface abrasion (and eraser residue) merited further investigation (Bernier 1997). Penley Knipe (1997) evaluated a selection of aqueous and nonaqueous surface-cleaning techniques on silver gelatin photographs. Using SEM at 310x, she observed the most pronounced changes on the surface of the ethanol/water-treated samples, which she described as appearing “dehydrated” (Knipe 1997, 24). Her results are based on cleaning techniques employed to remove only superficial surface grime and not silver mirroring as indicated by the presence of silver mirroring remaining on a sample after it had undergone treatment (Knipe 1997). The removal of silver mirroring typically requires a slightly more aggressive technique than that used for surface-cleaning.
The purpose of this two-part study was to examine selected treatment techniques while focusing on two types of potential treatment effects: (Part I) the immediate physical effects on the gelatin surface that might occur as a result of the removal of silver mirroring and (Part II) the long-term effects of the treatments, specifically the potential recurrence of silver mirroring. With regard to silver mirroring recurrence, the treatments were compared to determine whether any would promote or retard further formation of mirroring while samples were artificially aged.
The treatments used in this study were based on suggestions from practicing conservators and published studies describing successful treatments (Gann 1997). The treatments included were a mechanical cleaning method using PVC erasers, a combined aqueous and mechanical cleaning method using an ethanol/water solution, chemical treatment using an iodine/ethanol solution, and the application of a surface coating with methyl cellulose (MC) or wax.
In the first part of the study, SEM was used to examine the physical effects of treatment on the gelatin surface using naturally aged photographs with silver mirroring and freshly processed contemporary photographs, which were artificially aged to produce silver mirroring. For the second part of the study, UV-vis spectrophotometry was used to quantify the recurrence of silver mirroring on treated and subsequently aged samples. In order to examine the recurrence of silver mirroring under controlled conditions, only the freshly processed photographs were used to assure the results obtained were based on the treatments performed and would not be influenced by other factors of aging that might exist in the case of naturally aged photographs whose histories are unknown.

Figure 1: Outline of the experimental procedure followed in this study.
Four early twentieth-century, silver gelatin developed-out photographs were used for Part I of the study: two matte-surfaced photographs and two semi-glossy photographs. These historic samples were selected because they exhibited a moderate to extensive degree of mirroring without any obvious surface damages or accretions that would interfere with treatments. The photographs were unidentified family portraits collected from local Buffalo thrift shops. The markings on the photographs suggested they were created in the United States. In preparation for treatment, the photographs were cut into equal treatment sections that included controls.
Ilford Multigrade IV, fiber-based, matte-surfaced photographic papers were used in Part I and Part II of the study. A number of papers were exposed to create a uniform black image layer using a template to create a grid of squares each measuring 3/4 × 3/4 inches with 1/8 inch wide borders. The papers were processed using Ilford Multigrade paper developer, Ilford IN-1 stop bath, Ilford Multigrade fixer and Kodak hypo clearing agent as per manufacturer instructions. The papers were then washed for one hour in an archival washer and subsequently dried in a heated drum dryer. The samples were then hand-cut from each paper and housed between blotters until needed.
To create the silver mirroring, the freshly processed samples were incubated in a glass desiccator jar with an inside diameter of 150 mm and a capacity of 2 L under conditions based on those described in the ANSI peroxide aging test IT 9.15 (1997) used to test image stability. The test was modified for use with available equipment. This system produced an even layer of mirroring which was essential for quantifying the degree of silver mirroring recurrence.
A six-ply archival matboard was cut to fit into the bottom of the desiccator to provide a level surface on which to place the reagents. The board was encapsulated in Marvelseal, a moisture-proof heat-seal material, that prevented the board from absorbing vapors from the chamber. The desiccator contained a saturated salt solution made from 12 grams of potassium chloride and 5 mL of deionized water to maintain a relative humidity of approximately 80%. The potassium chloride salt solution was evenly distributed into six Mylar trays each measuring 1×1 inches. A 4.25 cm Whatman #2 filter paper saturated with 0.4 mL of 30% hydrogen peroxide was set on a watch glass that had been placed in the center of the trays. Kodak brown toner, 1.8 mL, whose primary component is potassium sulfide was distributed evenly between six of the Whatman #2 filter papers. The filter papers were placed resting on the edges of the Mylar salt trays. The addition of sulfur to the system was a modification suggested by Douglas Nishimura (1998) of the Image Permanence Institute, RIT, and was also used similarly in Nielson's study (1993). This was based on an observation made at IPI where silver mirroring could not readily be reproduced on freshly processed photographs unless they were also exposed to sulfur in the incubation chamber. The reagents were evenly distributed at the bottom of the desiccator to ensure their vapors would evenly evolve within the chamber and produce consistent incubation results.
Two layers of a polyester capillary matting and a layer of Pecap (76-T), a polyester monofilament screening material, were placed on a perforated ceramic tray supporting the samples to allow the chemical vapors to permeate evenly through to the samples. A double layer of Parafilm “M” was used to seal the opening at the top of the lid as well as along the rim to prevent any vapors from escaping from the desiccator. The desiccator was then placed in a Blue M humidity aging chamber for 17 hours, that had been preconditioned to 0% RH and 55°C. This system, under the same conditions, was also used to age the treated samples in Part II of the study where the recurrence of silver mirroring was examined.
The samples were treated using the following five methods to remove the silver mirroring without visibly damaging the surfaces.
SEM was used to study the surface of the photographs because it provided the high magnification, great depth of field, and high resolution that was necessary to detect any visual changes on the surface of the samples.
Samples from the four historic photographs and one freshly processed photograph were examined before and after treatment using SEM at 1000x and 5000x. The samples were coated with gold to make them conductive. Since the process of preparing samples for SEM is destructive, the samples needed for testing had to be removed from different but comparable image areas of the photographs.
In order to determine the degree of silver mirroring recurrence effectively, a means of quantifying this phenomenon was necessary. The formation of mirroring results in a change in image density owing to the silver ions migrating away from their original locations and forming an irregular layer on the surface of the print. Reflectance densitometers are commonly used to determine density changes in photographs as a result of image fading. The use of a reflectance densitometer to measure the degree of mirroring recurrence proved problematic since the reflective surface of the mirroring would read as a lower density and the difference between a thin layer and a slightly thicker layer could not be distinguished with this instrument. Also, the densitometer could only measure a very small surface area that would not be a good representation of the whole as the mirroring tended to form more heavily at the interface of nonimage and image areas. A Bausch & Lomb Spectronic 20 spectrophotometer, however, which is designed to measure diffuse reflectance at different wavelengths was found to be useful in quantifying the degree of mirroring. The instrument was used because it was sensitive enough to distinguish between subtle degrees of mirroring and could accommodate the entire sample surface at once.
For this part of the study only the freshly processed samples were used to maintain controllable conditions. Two sample sets were treated identically. The data for the two sets correlated very well and demonstrated repeatability under the conditions used in this study. The percent reflectance of the untreated mirrored samples and the treated samples was measured at 30 nm intervals across the visible spectrum. The samples were then aged in the desiccator under the same conditions used to create the mirroring on these samples. The percent reflectance of the samples was measured again, and the change in percent reflectance was calculated for each sample set. The instrument was calibrated using magnesium carbonate as the white standard to establish 100% reflectance while a black body was used for the black standard to obtain 0% reflectance.
Surface Examination Using SEM
PVC Eraser: The matte-surfaced samples were altered significantly as a result of treatment (fig. 2–7). The SEM micrographs of the surfaces of these photographs show they have a pronounced surface topography and suggest that it was abraded during the eraser treatment. It is possible that some of the matting agent particles closer to the surface were removed through abrasion along with some emulsion. Minute scratch marks that follow the direction of treatment were probably created by the abrasives contained in the eraser.
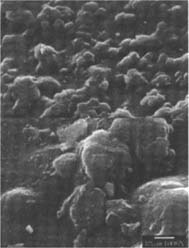
Fig.2
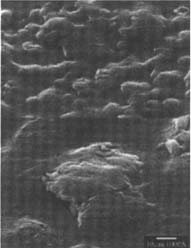
Fig.3
Figures 2-3: SEM micrographs of naturally aged, matte-surfaced sample A shown at 1000x and 5000x (2) before treatment and (3) after eraser treatment.
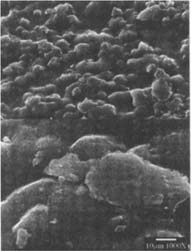
Fig.4
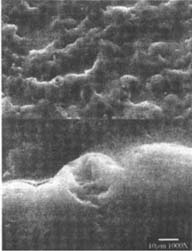
Fig.5
Figures 4-5: SEM micrographs of naturally aged, matte-surfaced sample B shown at 1000x and 5000x (4) before treatment, and with signs of abrasion and burnishing (5) after eraser treatment.
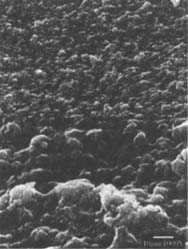
Fig.6
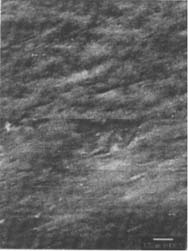
Fig.7
Figures 6-7: SEM micrographs of freshly processed, matte-surfaced sample E shown at 1000x and 5000x (6) before treatment, and (7) after eraser treatment.
The newly exposed surface had a burnished appearance. The burnishing was much more pronounced on the treated, freshly processed matte-surfaced samples (fig.7) then on the naturally aged, treated matte-surfaced photographs. Only one of the two glossy-surfaced samples (fig.9) showed scratches after treatment. The smooth surface of the glossy photograph made it difficult to determine whether or not these scratches were produced by the eraser because there were no other obvious signs of treatment such as the burnishing seen on the matte-surfaced samples.

Fig.8
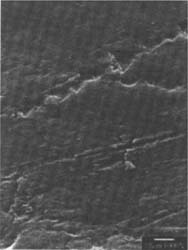
Fig.9
Figures 8-9: SEM micrographs of naturally aged, glossy-surfaced sample C shown at 1000x and 5000x (8) before treatment, and with some scratches (9) after eraser treatment.
Ethanol/Water solution: The matte-surfaced samples were altered significantly as a result of this treatment (fig. 10–11). These samples showed voids in the surface indicating that material had been removed from the emulsion during treatment.
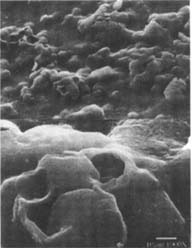
Fig.10
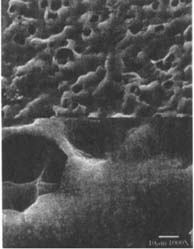
Fig.11
Figures 10-11: SEM of naturally aged, matte-surfaced samples (10) A and (11) B shown at 1000x and 5000x with voids in the surfaces after treatment with the ethanol/water solution.
The voids in the naturally aged matte-surfaced samples were larger than those found in the freshly processed matte-surfaced sample (fig.12).
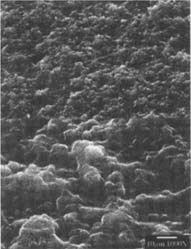
Figure 12: SEM micrograph of the freshly processed, matte-surfaced sample E shown at 1000x and 5000x with voids in the surface after treatment with the ethanol/water solution.
Judging by their size and shape, the large voids appear to correspond to the shape of starch granules, which might have been used as a matting agent and have indeed been mentioned in related literature (Reilly 1986). Starch granules may have been dislodged by the gelatin swelling in contact with water and by the simultaneous physical impact of the swab moving across the surface. A microchemical test using an iodine/potassium iodide solution was performed to confirm the presence of starch in the emulsion. Small samples of the emulsion layer were removed from untreated portions of the two naturally aged matte-surfaced photographs and subsequently tested with the solution. Upon testing, there was no obvious color change that would indicate the presence of starch. A color change, however, was difficult to discern under the stereomicroscope. Therefore, the results of this test were inconclusive.
The appearance of the voids in the freshly processed sample suggests a much smaller granular matter than starch had been removed. The nature of this material is most likely a matting agent or constituent of a matting agent, which may be silica or other materials used in contemporary photographic emulsions (fig.12). The surface of the glossy samples did not appear to have been adversely affected by the ethanol/water solution (fig.13).

Figure 13: SEM micrograph of naturally aged, glossy-surfaced sample C shown at 1000x and 5000x (13) with no obvious signs of alterations after treatment with the ethanol/water solution.
Iodine/Ethanol Solution: The gelatin surfaces of both the glossy and matte historic as well as the matte modern samples do not appear to have been disrupted by the chemical treatment as it left their surface topographies unchanged (fig.14–16).
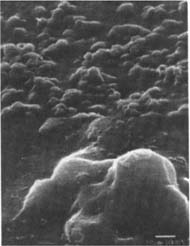
Fig.14

Fig.15
Figures 14-15: SEM micrographs of naturally aged, matte-surfaced sample A (14) and naturally aged, glossy-surfaced sample C (15) shown at 1000x and 5000x with no signs of damage to the surface after treatment with the iodine/ethanol solution.
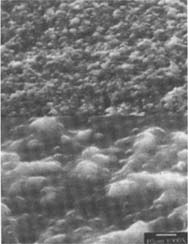
Figure 16: SEM micrograph of the freshly processed, matte-surfaced sample E shown at 1000x and 5000x after treatment with the iodine/ethanol solution.
Coating with Wax or MC: Because these coating treatments did not involve any removal of silver-mirroring, there was no disruption to the surface that could be seen with SEM. The coatings, however, did alter the appearance of the surface topography of the matte-surfaced samples by filling in the recesses thereby creating a smooth or leveled surface (fig.17–18).
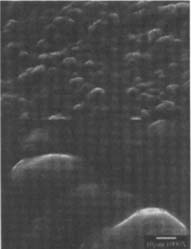
Fig.17
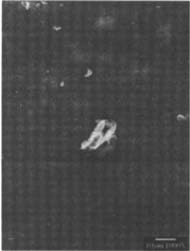
Fig.18
Figures 17-18: SEM micrographs of naturally aged, matte-surfaced sample B shown at 1000x and 5000x (17) after coating with methyl cellulose and (18) after coating with wax.
All treatment methods were quite effective in treating the silver mirroring. The coatings were more effective in eliminating the appearance of the mirroring on the matte-surfaced samples than on the glossy surfaced samples. The degree of effectiveness of the coatings, however, varied from sample to sample. Removing the mirroring mechanically with the eraser required the greatest physical effort. It is not surprising, therefore, that of all the treatments tested, only the eraser treatment caused alterations of the photograph surfaces detectable even without SEM. The contemporary matte-surfaced sample appeared a bit burnished and showed some scratches when viewed under low magnification. It was only the eraser treatment that showed side effects pronounced enough to allow us to correlate our normal visual observation of the surface with the SEM micrograph images.
Although swabbing with the ethanol/water solution caused voids in the gelatin emulsion that are rather prominent in the SEM micrograph images, these voids are undetectable under the optical microscope. Observing the treatment under the microscope at a low magnification did, however, reveal the immediate swelling of the dampened gelatin surface when treated with lightly moistened swabs. The swelling appears to have facilitated, in conjunction with the swabbing action, the loss of the starch granules and other granular matter believed to have been used as matting agents in the matte-surfaced samples.
Two general observations can be made about the iodine/ethanol treatment: it proved to be less easily controllable than any of the other three treatments tested; it was also the only treatment that caused an immediate alteration of image tonality. Image tonality increased in prominence with an increase in treatment duration, especially if the solution was able to penetrate quickly below the surface through cracks in the emulsion. This occurred more so with the historic photographs than with the freshly processed samples. The freshly processed samples, however, exhibited a delamination of the gelatin emulsion from the baryta layer in the non-image area when the samples were placed in the fixing solution, whereas the black image area was unaffected by this. The naturally aged samples which also contained a baryta layer remained unaffected by this phenomenon.
Examination of Silver Mirroring Recurrence Using Spectrophotometry
The results are illustrated in Figure 19. The graph shows the changes in percent reflectance that were detected by the spectrophotometer after the sample sets were aged. The figures represent the mean of the data collected from the two sample sets at each wavelength. The samples showed an overall increase in surface reflectance upon aging. In some cases, however, the samples coated with the MC or the wax exhibited a slight decrease in reflectance at certain wavelengths.
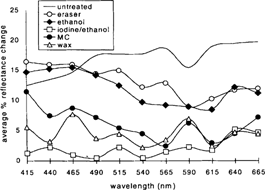
Figure 19: Graph illustrating average change in percent reflectance on aging for the two sample sets at corresponding wavelengths.
The samples treated with the iodine/ethanol solution experienced very little mirroring recurrence. Changes in surface reflectance across the visible range averaged an increase of 3% or less. The MC and wax coatings also appeared to inhibit recurrence to a certain extent. Surface reflectance across the visible range increased approximately 7% (+/− 1%). The ethanol/water and PVC eraser-treated samples exhibited more mirroring recurrence, ranging between approximately 10% and 17% increase in reflectance. The untreated control changed the most, ranging between 12% and 20% increase in reflectance.
It might not be so surprising to see that the coated samples did not exhibit much recurrence of silver mirroring because the coatings seal the surface of the photograph to a certain extent and prevent the further migration of silver to the surface, or, if there were any recurrence, it may not be visible through either of the coatings. The eraser and ethanol treatments removed the existing mirroring but left a fresh and slightly disrupted emulsion layer exposed that seems to be especially sensitive to the reformation of mirroring. The most interesting result is the almost complete lack of reformation of mirroring on the iodine/ethanol-treated samples. This suggests the chemical treatment was not only effective in removing existing mirroring, but it also seemed to have benefited the image stability of images subjected to the incubation conditions used in this study. These results correlate with observations made by Brandt (1984) and Nielson (1993) regarding the stabilizing effect of iodine on image silver. Brandt suggests a process by which iodide acts as a catalyst poison for the reduction of peroxide on silver by increasing the activation energy of the reaction (Brandt 1984).
Several observations can be made at the conclusion of this study: all treatments, carried out on small test samples, were more or less successful in treating the silver mirroring. It should be noted, however, that the application of these treatments in conservation practice warrants separate discussion. The results indicate that treatments involving some degree of physical manipulation of the surface may have detrimental effects on the surface topography of the more highly textured or matte-surfaced photographs while imparting little to no change to the surfaces of the smooth or glossy-surfaced photographs. The results also suggest the iodine/ethanol method appears to have a stabilizing effect on remaining silver image particles during subsequent aging. The results of the study also indicate different treatments might have an influence on the rate of silver mirroring recurrence.
ANSI. 1997. Methods for the evaluation of the effectiveness of chemical conversion of silver images against oxidation, ANSI/ISO 12206–1995, ANSI/NAPM IT-9.15–1997. In American national standard for imaging materials. New York: American National Standards Institute.
Bernier, B. 1997. A study of poly(vinyl chloride) erasers used in the surface cleaning of photographs. Topics in Photographic Preservation 7: 10–18.
Brandt, E.S. 1984. Mechanistic studies of stability. 2: Iodide adsorption on silver in the presence of thiosulfate and the influence of adsorbed iodide on the catalytic properties of silver toward hydrogen peroxide. Photographic Science and Engineering 28 (Jan/Feb): 13–19.
Gann, L. 1997. Silver mirroring. Unpublished document. Rotterdam: Nationaal Fotorestauratie-Atelier.
Hendriks, K.B., Hill, G., Iraci, J., Lesser, B., and B. Thurgood. 1991. Fundamentals of Photographic Conservation: A Study Guide. Toronto, Ontario, Canada: National Archives of Canada.
Knipe, P. 1997. A study of poly (vinyl chloride) erasers used in the surface cleaning of photographs. Topics in Photographic Preservation 7: 19–26.
Nielson, U.B. 1993. Silver mirror on photographs. Thesis project. Royal Danish Academy of Fine Arts, School of Conservation.
Nishimura, D. 1998. Personal Communication. Image Permanence Institute, Rochester Institute of Technology, Rochester, New York, 14623–5604.
Reilly, J.M. 1986. Care and Identification of 19th Century Photographic Prints. Rochester, New York: Eastman Kodak Company.
The authors wish to thank Dr. Peter Bush at the South Campus Instrumentation Center. University of Buffalo, for the SEM analysis and his significant contributions to the interpretation of the results. In addition, we would like to express thanks to Douglas Nishimura, Scientist. Image Permanence Institute, for suggesting the use of the ANSI peroxide aging test and for generously offering his expertise and kindly making himself available for questions. We would also like to thank Noelle Wiedner Science Department, Buffalo State College, for her assistance with the use of the spectrophotometer. And finally, special thanks is extended to F. Christopher Tahk, Director, Art Conservation Department, Buffalo State College, for offering his insight on silver deterioration and chemical treatment, providing support throughout the project and for reviewing the manuscript and making many valuable suggestions and contributions.