Topics in Photographic Preservation 2001, Volume 9, Article 7 (pp. 97-102)
By Niccolo Caldararo and Candis Griggs
Conservation Art Service P.O. Box 77570
San Francisco, Ca. 94107
Perhaps millions of slides have been made of art historical materials, archaeological sites and objects, scientifically important procedures, historical events and a vast number of other types of information of significance. A great number of these slides have entered into collections for a number of diverse reasons. The durability of the slide depends on the emulsion, carrier and slide frame. These materials have changed over time, from glass to plastics for the carrier and from metal to paper and plastic for the slide frame. In many collections varying humidity or accidents have caused damage to one or all of these components. Because of the fragile nature of these materials, the slides are often damaged beyond repair. One of the main agents of this damage is the growth of mold on the surface of the slide, specifically on the emulsion. Removal of this growth has been difficult without severe damage to the emulsion and image. There is little information in the literature to guide the curator or conservator in this problem. Our research has led us to the chitin building blocks of the mold and its essential relationship to the emulsion, considered in basic regard as digestion. Taking advantage of this relationship we have devised a method of removing the mold bodies which results in no or little observable increased damage to the emulsion and image.
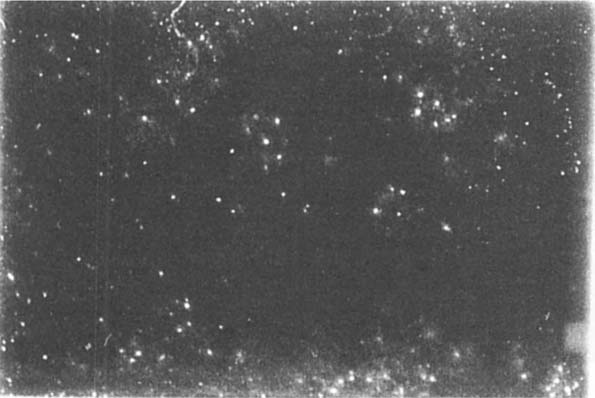
Removal of mold from slides has not been reported in the literature previous to this article (see figure 1). Our work began with a review of methods to clean the surface of photographic emulsions. We proceeded from manual methods, swabing, brushing, vacuuming to chemical methods that attempted to attack chemical bonds using solvents to attempts to develop methods designed to change the substrate to release the mold by pH change, to then, an examination of the nature of the digestion and attachment mechanism of the mold hypha. We were led in this endeavor by results reported by Shirk (1954). Shirk summarized studies conducted during WWII by government scientists and other researchers to clean mold damaged film, photographs and photographic materials. Shirk reports success in cleaning “slightly damaged” negatives using cloth saturated with alcohol or carbon tetrachloride. Many, if not most slide emulsions are damaged by the use of many alcohols and Shirk is not specific in his remarks. Carbon tetrachloride, however, is harmless to most emulsions but is also not effective in removing most colonies of fungi. Shirk reports the use of HCL and anhydrous sodium sulfate in water, a combination we found injurious in almost all cases and the immersion in formaldehyde and Kodalk. Stains and “markings” are said to have been treated using sodium chloride-permanganate bleaching. It became apparent that Shirk's reports were for negatives only, and these summaries were not associated with reliable outcomes.

Fig. 1
We tested a great number of solvents and solvent combinations in an attempt to produce a solvent system that would change the emulsion/fungi interface in a way that might release the mold without solubilizing the emulsion. Some of this work was built upon existing research in the solubility of chitin (Austin, 1984). We plan to pursue this work utilizing more recent research in chitin and chitosan solubility (e.g., Ravindra, 1998) and our knowledge of variations in emulsion characteristics of slide manufacture over the past 60 years. We wanted, however, to produce a non-toxic and easily accomplished method, so solvents which required hazardous materials, protective procedures for operators and incurred hazardous waste costs were avoided for the present. If no other means were possible we felt this route could be revisited. Degrees of success were defined – how much change of the emulsion surface or leaching of the emulsion's components would be acceptable? Could we remove only a small amount of the surface of the emulsion and remove the fungi? Almost all emulsions were sensitive to, or soluble with exposure to water and water/solvent dual systems captured our attention for some time as did varieties of polar systems without water. None of these were entirely satisfying although a 1% solution of sodium hydroxide applied with a swab to remove the mold and then quickly evaporated with a hairdryer produced some convincing outcomes. All of these attempts seemed to hinge on softening the emulsion sufficiently to remove the fungi colonies and some small amount of gelled emulsion. The usual result was a film of partly embedded chitin and softened emulsion. Partially deacetylated chitin can be obtained by alkaline hydrolysis of chitin (Pangburn, et al., 1984). All these systems failed to produce a clear separation between the chitin and the emulsion, leaving in most cases partly dissolves masses of one or the other embedded in a film.
Many of the slides which came to us for examination were very old, often records of archaeological or anthropological activities and study (see figure 2). Differences in carrier type, glass or plastic, frame, paper, metal or plastic and emulsion composition produced variables to treatment that demanded a comprehensive approach. Christine L. Sundt's 1989 summary of slide manufacturing history was very helpful in understanding the variety of materials used and construction problems, especially with glass slides. Most treatments had to be limited to physical reduction of mold colony bodies by vacuuming or brushing. This was not satisfactory to most clients whose purpose was often to utilize the information on the slides for scientific or other scholarly ends, and reduction of mold masses did not achieve the desired clarity.
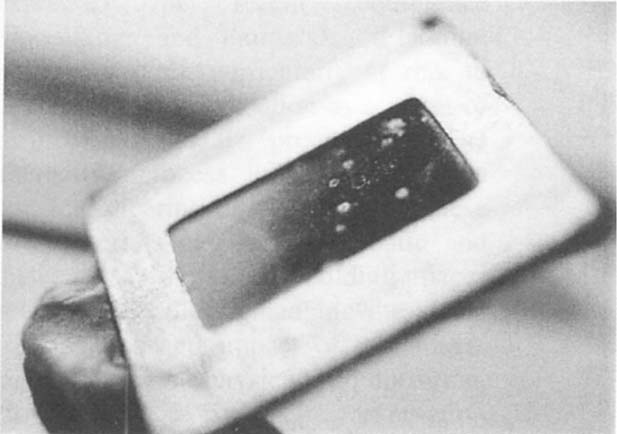
Fig. 2
These investigations led to a focus on the make up of the cell wall of mold. As Keeton (1980) notes, the cell wall of fungi is made up of chitin not cellulose. Chitin, like cellulose, is made up of condensation products, but unlike cellulose which is a condensation product of glucose, chitin is a condensation product of acetylglucosamine. It is a homopolymer, derived from glucosamine resulting in a polysaccaride with nitrogen. (Conn & Stuart, 1972). This chain can be attacked with acids which may explain the success reported by Shirk. Chitin is soluble in anhydrous acetic acid and 50% resorcinol. Focusing on chitin led us to attempts to discover how changing the attachment of the chitin might allow for its removal. First we tried enzymes that might selectively remove the chitin from the emulsion matrix. In most cases the enzymes required water as a medium for activity of the enzyme. Heard (1971) reported on experiments where successively higher concentrations of NaCl produced increased removal of chitin up to about 7%. This allowed for a medium with a reduced ability to gel the emulsion. The results of this single modality of treatment were not satisfactory, and in fact carried some significant problems due to the length of time required and the solubility of the emulsion and the limited range of activity of the chitinases available. Investigation into the physical properties of the emulsion and the fungi chitin lead to a discovery of an effective and acceptable method.
We decided to attempt some experiments aimed at physical attributes of the attachment and structure of the emulsion and chitin. Heat and cold were are first properties assessed. We found interestingly that freezing the slide produced a film of moisture on the surface of the emulsion which when wiped off carried away the fungi structures. The nature of removal varied considerably and complete success could only be routinely arrived at by using a swab in sub-zero temperatures and buffing the surface of the emulsion at room temperature (figure 3). The fungi had to be enveloped by the moisture for successful lifting and separation from the emulsion (figure 4). We theorized that the emulsion was actually frozen as was the chitin of the fungi, but they were physically separated by the moisture that formed on the surface. Later, it was found that Porter and Jaworski (1966) had suggested that freezing and thawing of chitin resulted in the release of an inhibitor from a synthetase or some hydrolytic enzymes which act upon the substrate which the chitin has formed on. This would seem unlikely as we irradiated the slides with UV according to food quality inactivation procedures for mold and neutralization of spores (Stevens, et al., 1997). At this time we are unsure if the removal success we have had with freezing is related to this or not. We also note that there is a significant amount of variation between some treated slides using this method. We are not sure if this is related to differ fungi species, to extent of damage to the emulsion surface, differences in time from removal from the refrigerator to the cleaning stand or simply operator error. We also find that removal of fungi is less effective in this subset by freezing but that the residue can sometimes be cleaned off with a solution of mineral spirits and water 1:1, mineral spirits and water and 1% NaOH, or a solution of 1 unit of Chitinase. All of these applied with a swab rubbed on and then rubbed off under a hairdryer.

Fig. 3
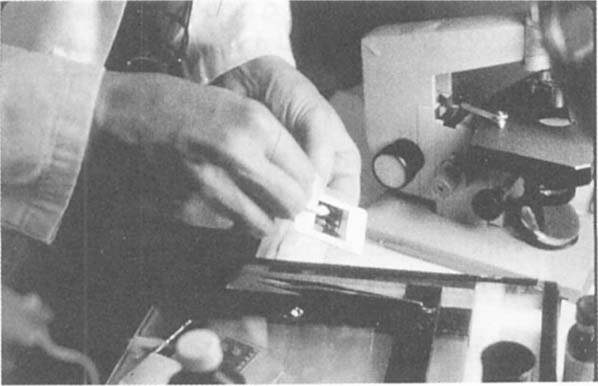
Fig. 4
Successful removal usually required the following procedure. The slides were placed on a plastic base in a refrigerator freezer at or below freezing temperature. The slides were removed when a film of moisture could be seen to have accumulated on the surface. They were then swabbed with a Q-tip removing all the moisture and fungi colonies with one tip and then buffing quickly the entire surface with the other (see before and after illustrations in figures 5a and 5b). Sometimes this had to be repeated two or three times. If the first removal was not done carefully, some chitin was deposited along the edges of the slide against the frame and could be seen as a margin in raking light. The initial cleaning is often so remarkable a change that the operator often believes the surface has been completely cleaned. Careful examination under raking light will often detect streaking which is a film of incompletely removed chitin in our opinion. Second or third attempts often remove this film adequately.

Fig. 5A

Fig. 5B
Our experiments and preliminary work indicates that this procedure can clear most slides of fungi colonies without detectable damage to the emulsion. The variation of removal needs to be studied to find it the variation is due to different species of fungi, variation in temperature at the workbench, extent of damage of the emulsion or operator error or variation. We also need to apply this technique in experimental conditions to a greater variety of slide type and manufacturer product over time. At present our results can be only applied to slides manufactured between the 1960s and 1990s. We do not recommend its use to slides older than this period. Slides treated thus far have mainly been Kodak products (Kodalux, etc.) and many unidentified types.
Austin, P. R., Chitin solvents and solubility parameters, Chitin, Chitosan and Related Enzymes, in J. P. Zikakis, ed., Academic Press, Orlando, 1984: 227–238.
Conn, E.E. and Stumpf, P.K., Outline of Biochemistry, 1972.
Heard, J. W., The degradation of Chitin by marine bacteria, Thesis SFSU Department of Biology, 1971.
Keeton, W. T., Biological Science, W.W. Norton, New York, 1980.
Pangburn, S. H., Trescony, P. V., and Heller, J., Partially deacetylated chitin: its use in self-regulated drug delivery systems, i Chitin, Chitosan, and Related Enzymes, J.P. Zikakis, ed., Academic Press, Orlando, 1984: 3–20.
Porter, C. A. and Jaworski, E. G., “The synthesis of chitin by particulate preparations of Allomyces macrogynus, Biochem., v. 5, 1966: 1149–1154.
Ravindra, R., “Solubility parameter of chitin and chitosan”, Carbohydrate Polymers, v. 36, n. 2, 1998: 121–127.
Shirk, H. G., Optical Instruments and Photographic Equipment, in G.A. Greathouse and C.J. Wessel, eds., Deterioration of Materials: Causes and Preventive Techniques, Reinhold, Publishing, N.Y., 1954: 702–746.
Stevens, C., Khan, V. A., Lu. J.Y., Wilson, C. L., et al., “Integration of Ultraviolet light with yeast treatment for control of postharvest storage of fruits and vegetables”, Biological Control, v. 10, 1997: 98–103.
Sundt, Christine L., Conservation Practices for Slide and Photograph Collections, VRA Special Bulletin Number 3, 1989.