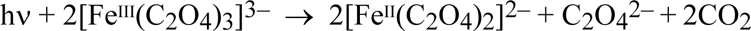
Topics in Photographic Preservation 2003, Volume 10, Article 2 (pp. 2-18)
Presented at the 30th AIC Annual Meeting, Miami, Florida, 2002
Sir John Herschel invented photographic contact-printing in Prussian blue in 1842, and named it ‘cyanotype’, devising both negative- and positive-working processes (Herschel 1842). The formulae he used are not cited explicitly in his publications, but can be found in his handwritten experimental memoranda relating to the period, which are held in the manuscript archive of the Harry Ransom Humanities Research Center, at the University of Texas at Austin (Ware 1999). At first, Herschel's invention was only taken up by amateur botanists for the purposes of plant illustration, most notably by Anna Atkins who, during two decades from 1843, produced her albums of botanical photograms in cyanotype, which have become highly-treasured items in the early photographic canon (Schaaf 1985, 1992).
Following Herschel's death in 1871, cyanotype was ‘re-invented’ by entrepreneurs who exploited its potential as a reprographic medium. The re-styled ‘ferroprussiate’ process of Marion and Company found some use among photographers as a cheap and easy option for proofing negatives, but its major market was for copying the plans in every drawing office. By the 1880s, it had become the chief process for photocopying, lasting until the mid-1950's, when it began to be displaced, first by the diazo print medium, then by the invention of electrophotography (Lathrop 1980; Kissell and Vigneau 1999; Price 2003). Even in obsolescence, the cyanotype process has endowed our language with an indelible new word: the blueprint.
In British photographic circles, cyanotype was not accepted as a pictorial medium until the last two decades of the 20th century, thanks to the intolerant responses of early critics to its powerful colour: a survey of the major British collections of photographic art reveals an almost total aesthetic boycott of the process until recent years. This prejudice did not prevail universally. Substantial holdings of cyanotypes by photographic artists such as Haviland, Le Secq, and Curtis exist in museums in France and North America. The process was also used for proofing reference images as may be seen in the Sambourne collection at Leighton House, London, and the Muybridge archive at the Smithsonian Institution, Washington DC. Possibly owing to the ready availability of the paper, cyanotype was used to document some significant engineering enterprises and topographical studies, such as railways (National Railway Museum, York, England), the construction of the Forth Road Bridge (Canadian Centre for Architecture, Montreal), the cutting of the Panama Canal (National Library of Australia, Canberra), and Henry Peter Bosse's survey of the River Mississippi (Neuzil 2001).
The chemical identity of Prussian blue is ferric ferrocyanide (iron(III) hexacyanoferrate(II) in modern nomenclature) containing iron in both the +3 and +2 oxidation states. Intense colour is a quintessential property of such mixed oxidation state metal compounds. The deep blue is due to an absorption band in the red region of the visible spectrum around 700 nm, caused by an inter-valence electronic charge-transfer transition (Robin and Day 1967).
The negative-working cyanotype process, which is by far the more successful and important, uses a sensitizer consisting of a soluble ferricyanide and a light-sensitive iron(III) carboxylate, such as ammonium ferric citrate or ammonium ferrioxalate (Ware 1999). The iron(III) complex is photodecomposed to give iron(II):
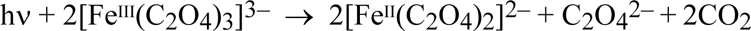
The complex iron(II) photoproduct is in equilibrium with the aquated ferrous ion:
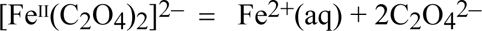
and this then reacts with the ferricyanide anion to precipitate the highly insoluble substance, Prussian blue:
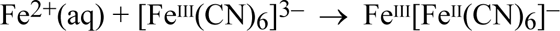
For clarity, throughout this account, the formula of Prussian blue will be written as the anion of the ‘ideal’ cubic structure, FeIII[FeII[(CN)6]-, where it is understood that the cation can be potassium or ammonium, depending on the circumstances of the preparation. The oxidation state of each iron atom in these formulae will be represented by superscripted Roman figures. Because this process entails the reaction of ferrous ion with ferricyanide, most technical accounts of cyanotype make the common error of describing this pigment, sometimes called Turnbull's blue, as ferrous ferricyanide. Recent research has conclusively shown this to be wrong; when formed, ferrous ferricyanide instantly rearranges by transferring an electron between its iron centres to give ferric ferrocyanide, Prussian blue, as indicated in Table 1.
Table 1 Varieties of complex iron cyanides.
| Reactant | Ferricyanides | Ferrocyanides |
| Ferric salts | Ferric ferricyanide | Ferric ferrocyanide |
| Prussian yellow (Prussian brown) Soluble; a powerful oxidant, easily oxidises water, etc., being reduced via green intermediates (Berlin green) to Prussian blue | Prussian blue (Berlin blue) Highly insoluble; most intensely coloured, and most stable of all possible products in this table, to which the others revert | |
| Ferric salts | Ferric ferrocyanide | Ferrous ferrocyanide |
| Turnbull's blue The same as Prussian blue. It is not the expected ferrous ferricyanide, which is very unstable and reverts instantly to Prussian blue | Prussian white (Everitt's salt or Williamson's salt) Insoluble and colourless, but readily oxidised by air (and other oxidants) to Prussian blue |
When Prussian blue is faded by light the colourless substance, ferrous ferrocyanide or Prussian white, is the product, see Table 1. It should be understood that this fading is not brought about by light alone: the transformation of Prussian blue to Prussian white is a reduction which must be accompanied by an oxidation of an electron donor:
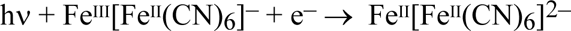
but what exactly is oxidised when a cyanotype fades is an unresolved question which will be addressed later. The formation of Prussian white also accounts for the tonal reversal that is seen during prolonged exposure of a cyanotype sensitizer. Provided the light-sensitive iron(III) salt is in excess, some of the Prussian blue is reduced to Prussian white by the iron(II) photoproduct, causing the cyanotype image to pale:
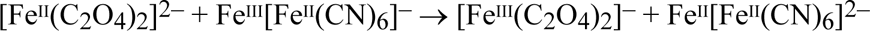
This tonal reversal in the regions of greatest exposure is the phenomenon of 'solarisation’ first observed and named by Herschel. After the exposure, the Prussian white is oxidised back to Prussian blue, either slowly by the oxygen of the air:
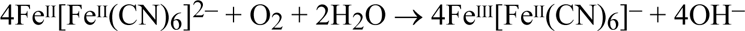
or more rapidly by including a bath of an oxidising agent, such as hydrogen peroxide, in the wet-processing sequence:
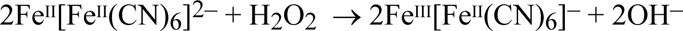
The chemistry of Prussian blue is complicated by the fact that its composition depends on its method of preparation (Sharpe 1976). The analytical variability of this family of substances has engaged - and frustrated - chemists for two centuries (Williams 1948), but only in the last two decades has a clear understanding begun to emerge (Ware 1999). The formulae for Prussian Blue are usually stated to range from an ‘insoluble’ Prussian blue, FeIIl4[FeII(CN)6]3, to a form that also contains potassium ions, KFeIII[FeII(CN)6], the so-called 'soluble’ Prussian blue. The latter is a serious misnomer: in fact, all forms of Prussian blue are highly insoluble in water. The apparent 'solubility’ in the latter case is an illusion created by the dispersion of the solid in water as colloidal particles, which form a blue suspension having the appearance of a true solution. The peptization of 'soluble’ Prussian blue is responsible for some of the problems that beset the making and conservation of cyanotypes.
The structural basis for all the Prussian blues is a cubic lattice of iron atoms, alternately ferric and ferrous; the cyanide groups bridge between them, with their carbon atoms coordinating the ferrous ions octahedrally. In a sense, Prussian blue is a chemical 'sponge’: the open channels in the lattice give it zeolytic properties, and the large void within each cube can accommodate and trap small molecules, eg water or metal ions, which accounts for some of the variations observed in the formulae found for the substance analytically. In the case of 'soluble’ Prussian blue, half of the cubic sites are occupied by potassium ions. The other cause of variable composition is the presence of lattice defects; the formula of the ‘insoluble’ variety is explained by the absence of one quarter of the ferrocyanide groups, with water molecules in their place (Buser et al. 1977).
The chemical properties of Prussian blue identify three distinct directions in which cyanotypes are vulnerable: to photochemical reduction, alkaline hydrolysis, and aqueous peptization. These pathways of destruction may conveniently be called ‘fading’, ‘bleaching’, and ‘dispersing’, respectively. Each leads to a loss of Prussian blue from the image, but each is chemically distinct in its causes, products, and remedies, which are summarised in Table 2.
Table 2 Three pathways of destruction for cyanotypes.
| Fading | Bleaching | Dispersing | |
| Description | Photochemically-induced reduction | Alkaline hydrolysis | Aqueous peptization |
| Cause | Visible and UV light and a reductant | Any substance of alkaline pH (>7) | Water and high ionic strength solutions |
| Product | Prussian white (ferrous ferrocyanide) | Hydrated ferric oxide and ferrocyanide ions | Colloidal sol of Prussian blue in water |
| Reversibility | Reversible by air in the dark | As ferric oxide ages, becomes irreversible | Pigment irreversibly lost from image |
Cyanotypes and commercial blueprints have acquired a reputation for fading partially in the light, but the extent has not been properly quantified (Reilly 1986). Densitometric measurements on facsimile cyanotypes exposed to daylight found a ‘loss of density ranging from 4% after 15 minutes exposure to 10% after 45 minutes exposure’ (Moor and Moor 1989). Monitoring of historic photographs at the National Gallery of Canada has been described (McElhone 1993); these studies include original cyanotypes by Atkins (1854) and Curtis (1868). These were illuminated by 50–60 lux incandescent tungsten, filtered to remove the UV. Densities were measured at intervals during periods of two to four years display in glazed mounts. No significant effects (ie no permanent density changes δD ≥ ± 0.02) were observed as a result of accumulated exposures of about 100 kilolux hours.
In the present work with facsimile material, the intention was to establish the levels of damage brought about by the three main pathways of vulnerability. Five different cyanotype formulations were tested, the first four of them representing the main categories of sensitizer used historically for the negative-working process; for brevity and ease of reference they will be allocated the names Smee, Herschel, Lietze, Valenta and Ware which are defined in Table 3.
Table 3 Formulae for varieties of cyanotype tested: % in mixed sensitizer.
| Name | Description | Ammonium ferric citrate | Potassium ferricyanide |
| Smee | Herschel's first process | none | 16% w/v |
| Herschel | Herschel's ‘standard’ recipe | 7% (brown) | 12% |
| Lietze | Typical C19th recipe | 10% (brown) | 8% |
| Valenta | Typical C20th recipe | 13% (green) | 6% |
| Ware | New cyanotype formula | 16% (ferrioxalate) | 10% (ammonium) |
For chemical purity and consistency, the paper generally used for coating was Atlantis Silversafe Photostore (Moor and Moor 1990), of weight 120 g/m2. Comparisons were also made with gelatin-sized papers, both contemporary (Fabriano 5 HP, and Arches Aquarelle HP) and of the type used 150 years ago (Whatman's Turkey Mill 1840 and J. Whatman 1849, made by Hollingworth and Balston, respectively). Coating was carried out with a glass rod, and the sensitized papers were all dried at room temperature in the dark.
Specimens to be tested were made by conventional cyanotype printing in direct sunlight, using a glazed printing-out frame with a hinged back, mimicking as accurately as possible the manner of exposure that would have been employed in the last century. The sun-prints were also compared with those made by an artificial ultraviolet light source, which is now universally used, employing four Phillips TLADK30/05 coated fluorescent tubes (having a maximum emission at 360 nm) at a distance of 8 cm. Calibrated step tablets were used as negatives for the test prints.
The light source used to induce fading was a commercial luminaire fitting, equipped with four 20 watt artificial daylight fluorescent tubes, General Electric type F20W/AD. Two cooling fans ensured that the temperature during exposure was maintained at 19 ± 1 °C and the relative humidity at 50 ± 5 %. The illuminance at the plane of the samples, measured with photographic exposure meters, was approximately 4 kilolux. Optical densities were measured to a precision of 0.002 by means of an X-Rite model 310 densitometer in diffuse reflectance mode, which was recalibrated by reference to a standard density plaque, with an accuracy of ± 0.01. All densities recorded refer to the ‘red’ channel of the reflectance colour head, corresponding to the wavelength of maximum absorption by Prussian blue.
A typical characteristic (D/logH) curve for the Herschel cyanotype sensitizer is shown in Fig. 1, where the curve is also re-plotted after an exposure to artificial daylight of ca two kilolux hours. All the other types of sensitizer suffered a similar loss of density, amounting to 0.1 - 0.2 in consequence of the light exposure.
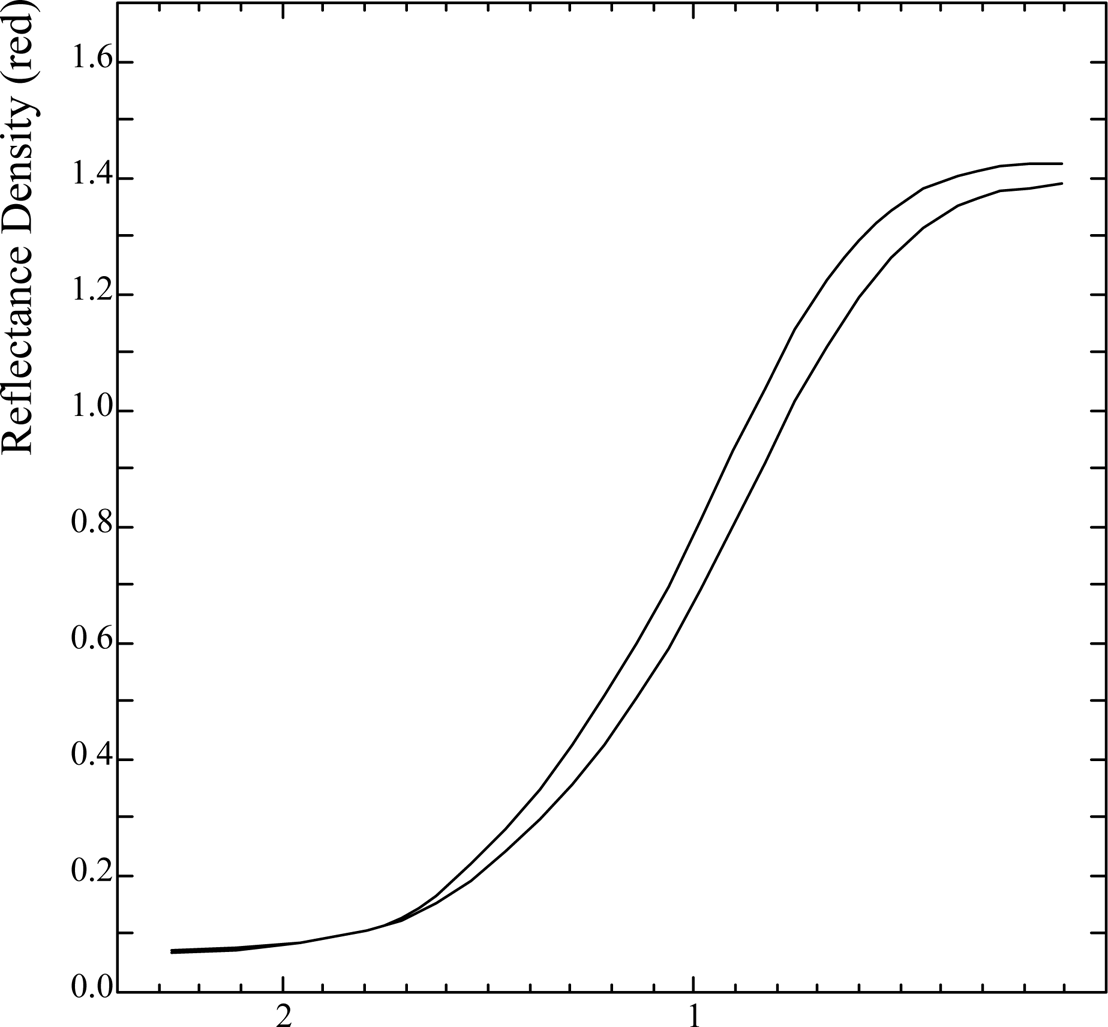
Fig. 1 Effect of fading exposure on characteristic curve for Herschel cyanotype sensitizer.
If Di represents the initial density of any step of the pigment layer in a step-tablet test, and Df is the density of the same step measured immediately after a fading exposure, the extent of fading is denoted by the difference:
It will be convenient to use a rounded parameter, called the fade, defined as 100AD for the discussion that follows.
Fig. 2 plots 100δD against the initial reflectance density, Di, to show how the fade varies across the tonal scale for the Herschel sensitizer. It reaches a broad maximum in the mid-tones and falls off towards both ends of the scale, showing that the fade is not proportional to the concentration of Prussian blue - which suggests that fading is not an intrinsic property of the substance.
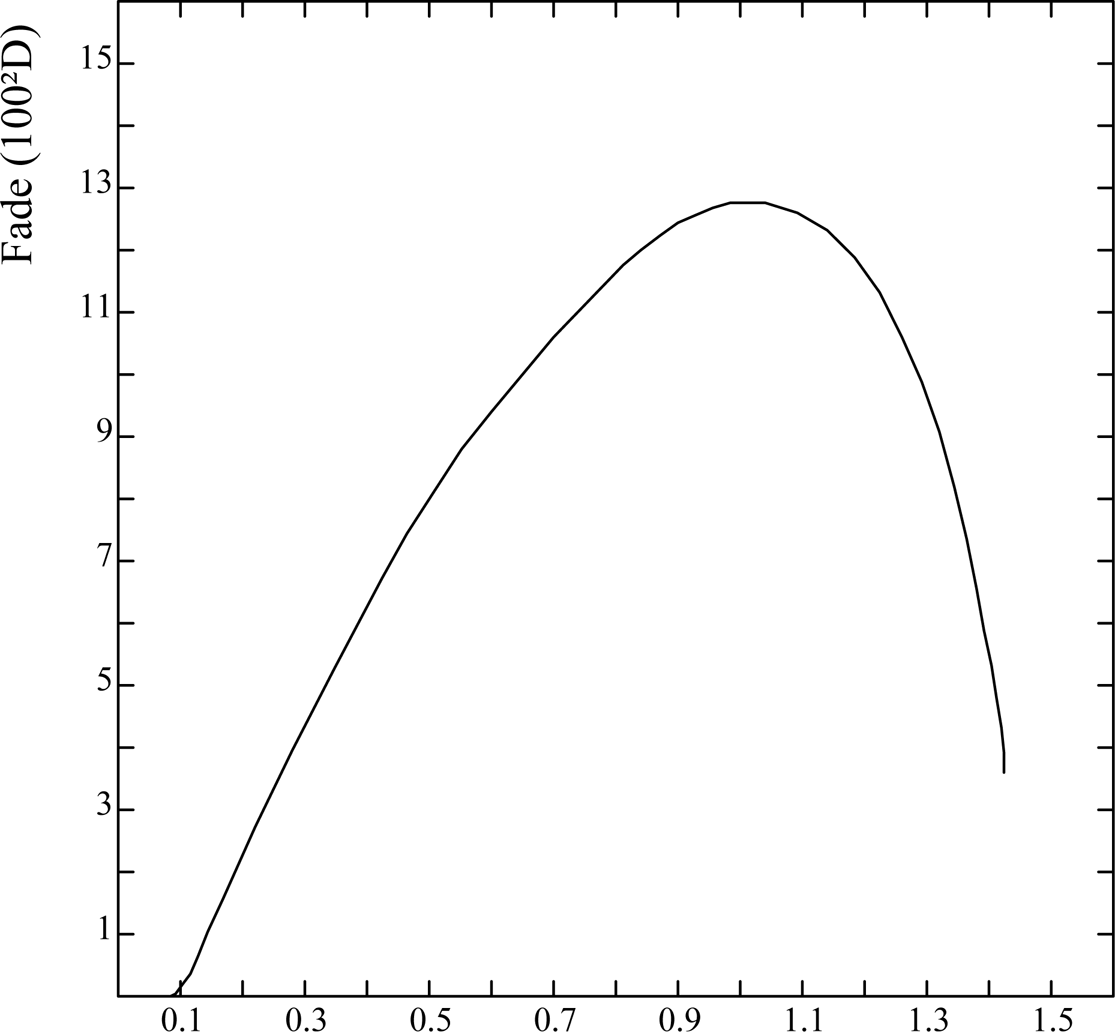
Fig. 2 Variation of cyanotype fading with initial density.
The maximum value of the fade for a given exposure is a convenient parameter to express the measured susceptibility of a particular Prussian blue layer. Values of the maximum fade for all the sensitizers are summarised in Table 4.
These results emphasise the importance of carrying out cyanotype fading studies on mid-tones, where the loss of picture information will be greatest, and not just on a region of maximum density, where there may be a large ‘reserve’ of Prussian blue in which little change will be observed. If the original image has a high contrast, with few intermediate tones, then the visible fading effect will be confined to the extremes of the tonal scale, where it is minimal. This is particularly the case with ‘line’ images, such as contact prints from engravings, or photograms of opaque objects. This may explain the wide disparities between ‘anecdotal’ reports of the fading - or otherwise - of cyanotypes.
Table 4 The maximum fade of cyanotype sensitizers exposed to 4 kilolux.
| Sensitizer type | Maximum fade 100δD | Initial density range Di |
| Smee | 13 | 0.90–0.95 |
| Herschel on aquapel-sized paper | 11 | 0.55–1.05 |
| Herschel on gelatin-sized paper | 12 | 0.80–1.20 |
| Lietze | 10 | 0.65–0.85 |
| Valenta | 10 | 0.35–0.85 |
| Ware | 22 | 0.85–1.15 |
Test strips were exposed in the artificial daylight source and selected steps densitometered at regular time intervals. When the densities reached steady values, the samples were removed to dark storage at ambient temperature and humidity (in the range 18 ± 2 °C and 50 ± 10% RH), with full access to the atmosphere, and their densities remeasured periodically.
Typical results are shown in Fig. 3 for the step that gave rise to the maximum fade of the Herschel sensitizer.
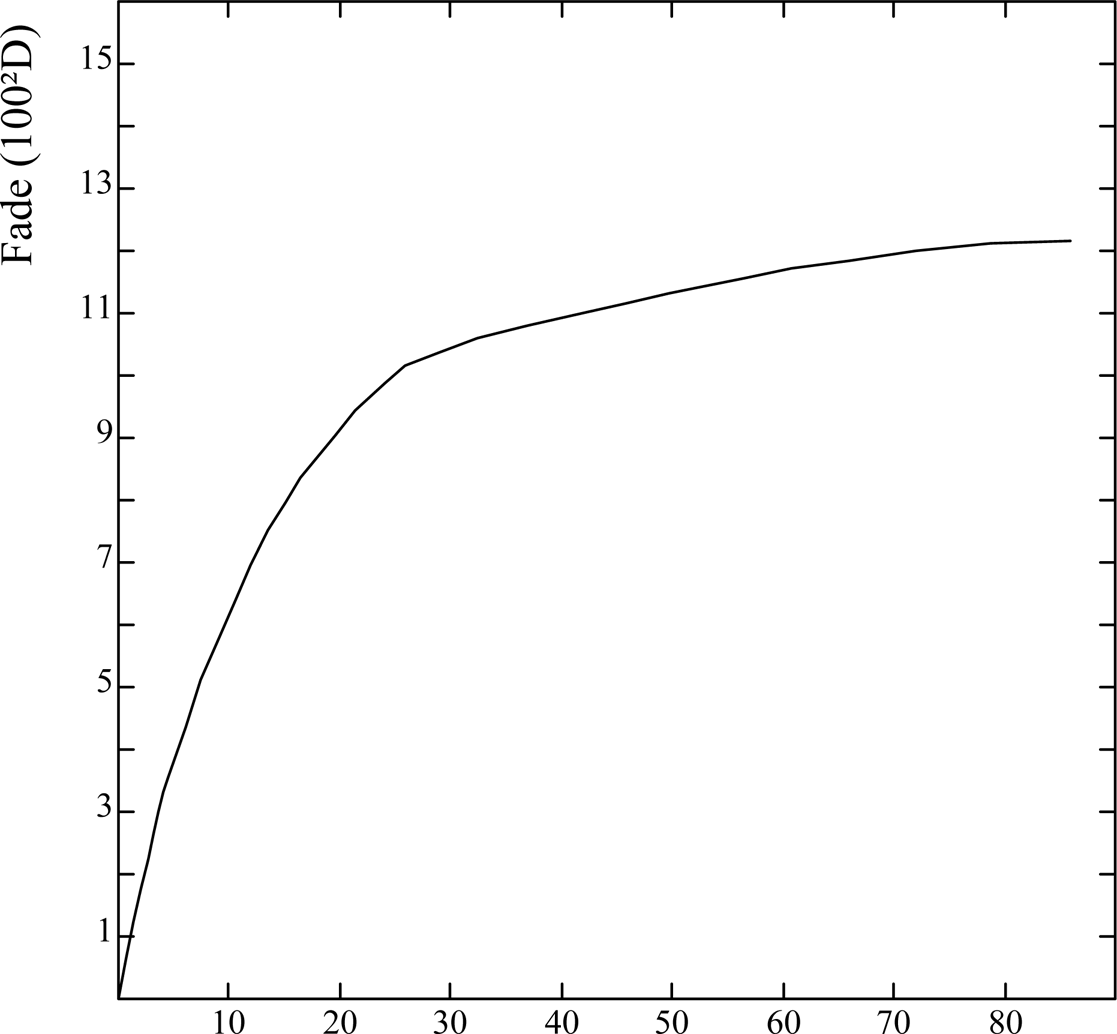
Fig. 3 Variation of Herschel cyanotype fading with exposure time at 4 kilolux illuminance.
The fade approaches its limiting value after only about one hour's exposure to four kilolux. The regain of density in the dark was usually at least 95% within one day, and 99% within five days or more, ie it was complete within the limits of experimental error. To test the reversibility of the fading, samples were subjected to repeated exposure and dark regain. Fig. 4 shows schematically the fluctuating density of one step over five such cycles in which the fading exposure was about four kilolux hours, and the regain period before re-measurement was several days. Had there been any permanent fade of the same order as the experimental uncertainty (ca. 1 unit) this would have built up progressively over five cycles of fading and regain to give an observable loss - which is not apparent.
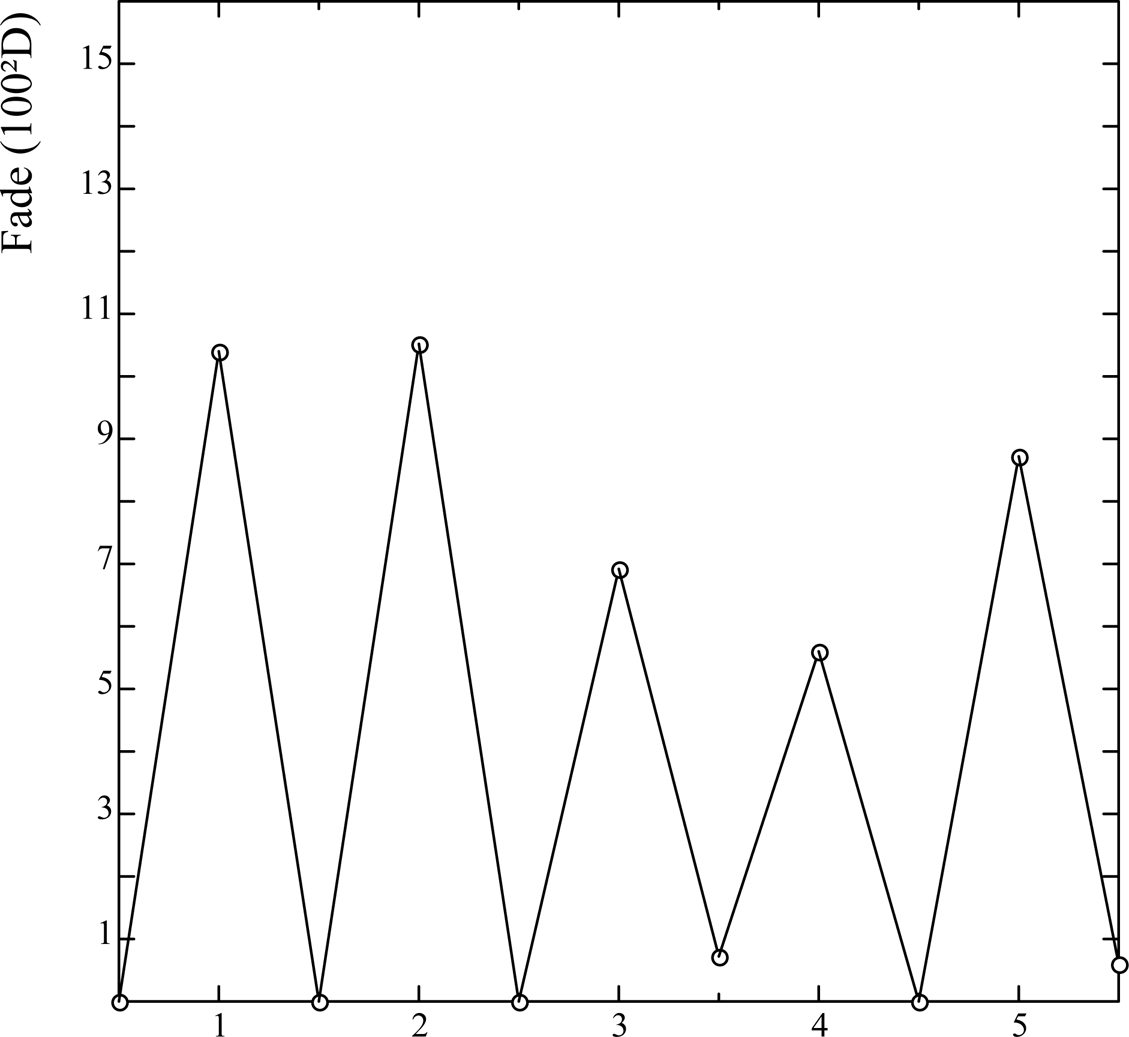
Fig. 4 Reversible fade and regain of Herschel cyanotype over five cycles of exposure.
For a given exposure, it is apparent from experimental evidence that the maximum fade depends on the illuminance. At low light levels (ca 50 lux), McElhone's data on monitoring of cyanotypes shows that an exposure totalling 100 kilolux hours causes no detectable fading. The probable reason for this is that the rate of density regain, due to air reoxidation of the Prussian white, is superimposed on the fading, and when the illuminance is so low that the rate of fading is less than or equal to the rate of regain, no fading at all will be observed, provided sufficient air can diffuse in to the vicinity of the print. When the illuminance is much higher, such as daylight at ca 4000 lux in the present tests, an identical exposure of 100 kilolux hours, which is reached in 25 hours, is found to incur a maximum fade of between 10 and 20, depending on the sensitizer. Finally, to determine the effect of very high illuminance, samples were exposed to direct sunlight, ca 100 kilolux, for about one hour, which caused a deep fade, of 40 or so. Thus, the same exposure of 100 kilolux hours, delivered at three levels of illuminance, has three different outcomes in the degree of fading of cyanotypes, showing that the phenomenon does not obey the photochemical Law of Reciprocity. Recently, J. Dunbar performed an experiment that confirms this view. Cyanotype specimens were enclosed in an anoxic environment (a Marvelseal™ packet containing Ageless™ oxygen scavenger) and given a 24 hour fading exposure, which caused them to turn totally white. When opened and exposed to the air, they regained their full blue colour (Dunbar 200?).
The fading process is a light-induced reduction of Prussian blue to Prussian white, which necessarily entails the acquisition of an electron from some other substance, and an extra cation to balance this increased negative charge. The question follows: what electron donors are there in a cyanotype print? The candidates fall into three categories which will be examined in turn:
1. The inherent stability of Prussian blue to light. The light-fastness of Prussian blue is described as ‘excellent’ (The Colour Index 19??) and manufacturers of artists’ pigments list it as ‘durable”. A fading test on the pigment showed no significant density loss after a total exposure of over 40 megalux hours (Kirby 1993). A sol of pure Prussian blue exhibits no tendency to decompose under prolonged irradiation with visible light, establishing that it does not significantly photo-oxidise water to oxygen, or its own bound cyanide ion (to form cyanate or cyanogen) under these conditions (Christensen et al. 1985).
2. Possible effects of constituents of the substrate and binder. In a cyanotype print, there are potentially oxidisable substances within the paper: primarily the cellulose substrate, any lignin, sizing agent, or other manufacturers' additives. Their possible contribution to fading was checked by making cyanotype test prints on a variety of non-oxidisable absorbent substrates: fibreglass filter paper, glass plates coated with silica or alumina (as used for thin-layer chromatography), and bisque-fired ceramic tile bodies. The general observation in all these tests was that cyanotypes formed on such non-reducing substrates faded in light as rapidly as those prepared similarly on pure cellulose paper. Thus, cellulose itself is not implicated as a major contributor to the fading process in cyanotype images. The possible effect of lignin has not been tested.
3. Incorporated impurities that destabilise Prussian blue. The most likely origin of light- sensitivity in cyanotypes is the incorporation of impurities deriving from the sensitizer. To test this, pure Prussian blue coatings were prepared on paper by applying successive coats of potassium ferricyanide and ferrous sulphate solutions. When dry, half the area was coated with a 10% solution of an oxidisable organic anion such as citrate or oxalate, and allowed to dry again. The density of the impregnated Prussian blue was compared with the control region after a period of dark storage to ensure that there had been no thermal reduction reaction, then the whole sheet was given a standard daylight exposure. The coated area was found to fade much more deeply than the control area, showing that these oxidisable organic anions do indeed render Prussian blue sensitive to photoreduction.
If the products of oxidising the impurity are lost from the cyanotype (for example as carbon dioxide gas, in the case of oxalate), the impurity should be progressively destroyed as light exposure accumulates:
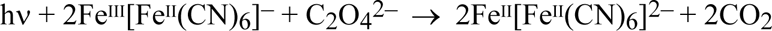
The Prussian white will still be fully re-oxidised by air, so the quantity of impurity should diminish with successive cycles of fading and regain, as the impurity is ‘burnt out”. This behaviour is quite marked in the Ware cyanotype, which is based on an oxalate sensitizer.
Of the many toning procedures suggested for cyanotypes, most de-stabilise the image, and cannot be recommended. In the lead toning method of Oscar Bolle, the processed cyanotype is immersed in a 5% solution of lead(II) acetate, preferably at a pH between 7 and 8, which shifts the blue towards a beautiful deep violet. Specimens treated in this way were found to exhibit improved resistance to light fading, by a factor of about four. Analysis by X-ray spectrometry strongly suggested that the lead(II) ions are incorporated in the Prussian blue lattice. However, the strong colour shift and the toxicity of the lead acetate bath debar this as a conservation procedure, but artists originating cyanotypes might well consider incorporating it into their practices.
Hydroxide ions rapidly decompose Prussian blue to hydrated ferric oxide, FeO(OH) and ferrocyanide ions:
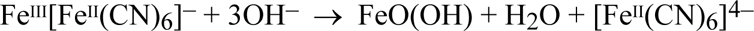
An alkali, such as a 0.25 molar solution of sodium carbonate with a pH of ca 10.7, destroys the Prussian blue of a cyanotype in less than half a minute. It has been reported that a buffer even at pH 9.4 takes only one to ten minutes to decolourise Prussian blue, depending on the method of its preparation (Holtzman 1945). This moderately alkaline pH is the same as a saturated solution of calcium carbonate (containing ca 0.001 g per 100 cc water); calcium carbonate is the buffer commonly incorporated in archival papers and boards. To test the extent of the danger from chalk buffers, processed cyanotype specimens of all five sensitizers were partially coated with a paste of calcium carbonate powder and distilled water, leaving a ‘control’ area for comparison, and housed in a high humidity environment (RH ≥ 95%) to keep the paste moist. Within 24 hours, there was a serious density loss (100δD ≈ 40 to 60) in the chalk-coated areas of all the sensitizers, compared with the adjacent ‘control’ areas.
Holtzman has sought treatments to protect the Prussian blue from this type of deterioration. He especially recommends the incorporation of nickel(II) ions into the pigment as a means of improving resistance to alkaline attack, by immersion in a bath of a nickel(II) salt. Holtzman found that Prussian blue so treated was ‘stable indefinitely’ at pH 9.4 (or, at least, for four months - the duration of his work), and it could even withstand the pH 10.7 solution of sodium carbonate for four to five hours (Holtzman 1945).
Holtzmann's nickel treatment was tested on Herschel and Ware cyanotypes. The process of soaking a cyanotype test strip in a solution of 10 % w/v nickel(II) sulphate for one hour causes a perceptible shift in the hue towards a more greenish-blue. Unfortunately, the treatment is also accompanied by a measurable loss of density from the mid-tones, presumably due to peptisation of the pigment: the loss was 10 from the Herschel specimen after one hour, and 15 after a 25 hour soak at room temperature; the corresponding losses from the Ware specimen were somewhat greater: 16 and 21, respectively. These specimens were tested for their resistance to alkali by immersing them in a buffer solution at pH 9.4 for 10 minutes, along with control specimens that had not been treated. All test strips were briefly rinsed in tap water and then dried and re-measured. The losses (100δD) are set out in Table 5, showing that the nickel(II) treatment is quite effective in protecting the Prussian blue of a cyanotype from alkaline degradation at this pH.
Table 5 Effects of alkaline hydrolysis at pH 9.4 for 10 minutes.
| Sensitizer type | Initial density | Loss (100δD) | Loss % |
| Smee | 0.953 | 58 | 61 |
| Herschel | 1.355 | 68 | 50 |
| Herschel treated with Ni(II) for 1 hr | 1.352 | 6 | 5 |
| Herschel treated with Ni(II) for 24 hrs | 1.122 | 5 | 5 |
| Lietze | 0.952 | 65 | 68 |
| Valenta | 1.322 | 94 | 71 |
| Ware | 1.086 | 65 | 60 |
| Ware treated with Ni(II) for 1 hr | 0.929 | 12 | 13 |
| Ware treated with Ni(II) for 24 hrs | 1.335 | 4 | 3 |
The tendency of Prussian blue to peptize causes a major problem for the traditional cyanotype process; image substance washes out of the paper during wet processing, which necessitates heavy over-exposure (as much as three or four stops) to compensate for the loss (Crawford 1979). Conservators have reported that cyanotypes washed for 15 minutes in tapwater (pH 7.5–8.5) lost about 18% in image density; in distilled water (pH 6–6.5) the loss was 4%; in deionised water (pH 6.3–6.6) the loss was said to be 0.00% (Moor and Moor 1997). In another study, washing cyanotypes in neutral pH tap-water or in de-ionized water at pH 6 for a period of 1.5 hours caused a marked loss of pigment (Wagner 1991).
The effects of aqueous washing on five different varieties of cyanotype were tested in the present work. For each process, a set of four nearly identical test strips was immersed in a gentle flow of tap water at 20 ± 2 °C, the pH of which was monitored periodically and found to be 7.2 ± 0.1. The strips were removed successively from the washing process after time intervals of five minutes, twenty minutes, one hour, and four hours; air-dried, and their densities remeasured. A similar test was performed in a two litre static bath of purified water at 22 °C, having a pH of 6.5. This ‘purified’ water was purchased from a retail pharmacist, and fulfilled the standards of purity set down by the British Pharmacopoeia. It was thought more realistic to use this, rather than de-ionised or distilled water, which vary in their origins, availability and quality.The results are summarised in Table 6, which shows that the rates of loss (100δD) for the different formulae vary between 1 and 4 units per ten minutes of immersion. Losses in the static bath of purified water were significantly lower.
Table 6 Effects of aqueous washing (Loss = 100δD).
| Sensitizer Type | Water quality | Initial density | Loss in 5 mins | Loss in 20 mins | Loss in 1 hour | Loss in 4 hours |
| Smee | Tap | 0.90 | 3 | 7 | 13 | 28 |
| Smee | Pure | 0.93 | <1 | 4 | 6 | - |
| Herschel | Tap | 0.95 | <1 | 2 | 6 | 14 |
| Herschel | Pure | 0.96 | <1 | <1 | 2 | 3 |
| Lietze | Tap | 0.98 | <1 | 3 | 7 | 18 |
| Lietze | Pure | 0.90 | <1 | <1 | 2 | 2 |
| Lietze gelatin | Tap | 0.83 | 3 | 8 | 14 | 31 |
| Lietze gelatin | Pure | 0.70 | 6 | 16 | 31 | 80 |
| Valenta | Tap | 0.86 | 1 | 4 | 10 | 31 |
| Valenta | Pure | 0.81 | <1 | 1 | 2 | - |
| Ware | Tap | 0.77 | 1 | 5 | 14 | 29 |
| Ware | Pure | 0.95 | <1 | 1 | 2 | 2 |
The concept of threshold exposure was introduced with the aim of providing a useful parameter for curators and conservators to discuss, in a semi-quantitative way, the vulnerability of cultural objects to light (Ware 1994). The threshold exposure results in one Just Noticeable Difference (JND) in any significant area. It defines the exposure at which ‘perceptible damage’ begins to accrue, as judged by the unaided human eye under good illumination, and is measured in the conventional units of exposure, lux seconds or, more conveniently, kilolux hours.
The psychometrics of human vision find a density difference between adjacent areas in the order of 0.01 to be ‘just noticeable’ under good viewing conditions. The notion of ‘significant area’ is somewhat subjective, but could be taken to be in the region of 1 mm2 upwards. One JND, of about 0.01 density units, corresponds to a fade of 1 unit. The experimental results above have shown that a maximum fade of ca 10 to 20 JNDs is brought about by an exposure of two to four kilolux hours, implying that the threshold exposure is only ca 200 lux hours at a level of illuminance of four kilolux. This may seem an alarmingly small exposure to cause perceptible change; it is comparable with the threshold exposure values observed for photogenic drawings, which are deemed too light-sensitive for exhibition under any conditions (Ware 1994). However, their behaviour is quite different from that of cyanotypes, because the fogging of a photogenic drawing is irreversible, cumulative and permanent, but the fading of a cyanotype (caused by moderate daylight exposure) is completely reversible by air re-oxidation. Moreover, as has been explained above, the failure of reciprocity in the case of cyanotypes means that the duration of exposure at 50 lux needed to fade them by even one JND may be very long indeed, if not infinite, and it is quite safe to exhibit them at, or near, this level of illumination. McElhone's findings offer a valuable reassurance to curators and conservators that historic cyanotypes can be safely displayed under proper conditions.
The extreme sensitivity of cyanotypes to alkali dictates that mildly acidic conditions are desirable for their storage, even though this is not considered beneficial for the paper substrate. From the evidence cited above, there can be no doubt that Prussian blue is destroyed by mild alkali (pH 8 to 10) much more rapidly than cellulose paper is destroyed by correspondingly mild acid (pH 6 to 4). Cyanotypes are best preserved in a slightly acidic, oxidising environment. Confronted with this dilemma, conservators should be prepared to subordinate their natural concern for the paper substrate to the best interests of preserving the image upon it. Any sort of de-acidification treatment is out of the question for cyanotypes. In view of the high permeability of plain paper, and the ease with which alkaline species can diffuse through it, no adhesives whatsoever should be applied to the verso of a cyanotype.
Treatment with nickel(II) confers considerable resistance to alkaline hydrolysis, as has been shown, but it is accompanied by a perceptible colour shift and some image loss; so its use as a conservation procedure would be highly questionable in most cases. The possibility of using this treatment deserves further investigation to see if the ‘cure can be made less harmful than the disease’, and, once again, it is a procedure that could be recommended for consideration to the originators of cyanotypes.
The thoroughness of washing a freshly-made cyanotype has a considerable effect on its susceptibility to fading. A Ware formulation cyanotype which had only received a wash of four minutes before drying suffered a maximum fade of 42 under a two kilolux hour exposure. In contrast, a similar test-piece washed for 20 minutes experienced a maximum fade of 16 under the same exposure. This provides further evidence that fading is promoted, in large part, by impurities left in the print as a result of imperfect processing. However such impurities cannot usually be fully washed out retrospectively, especially in old specimens, and prolonged washing in water itself can cause considerable peptization of the Prussian blue, which can pass unnoticed until the damage is done, so a compromise must be reached. Even simple aqueous washing should be avoided because of the risk of image loss; one should have a very good reason before washing a cyanotype. If it is considered essential for preservation, then the use of static baths of purified water is preferred, but the best option may be treatment with a ‘damp pack’: at least then one can keep a sharp lookout for any signs of transfer of the Prussian blue to the contact surface. For those making cyanotypes today, some precautions taken in their preparation could improve their archival qualities. Washing of the print should be thorough so that there is no trace of residual sensitizer. The Ware method recommends development in very dilute acid, which will destroy any undesirable alkaline buffer that may be present in the paper and ensure that the print is left in a condition favourable to the Prussian blue, possibly by the incorporation of acid cations into its lattice. The presence of the hydronium cation, H3O+, in the zeolytic voids will defend the Prussian blue against alkaline hydrolysis, while not presenting a direct threat to the cellulose of the paper. If a shift in colour towards greenish-blue is not unacceptable to the maker, then treatment with nickel(II) solution might also be incorporated in the processing as a means of fortifying the image.
It is now generally-accepted conservation practice that cyanotypes should not be mounted on, or stored in alkaline-buffered materials. Calcium carbonate clearly poses a threat to cyanotypes when in direct contact with the image; but it has little ability to migrate through cellulose, so the dangers of chalk-buffered enclosures can be overstated. It seems prudent, however, to continue the use of unbuffered materials for the mounting or wrapping of cyanotypes, where direct contact is involved.
It is desirable that cyanotypes which receive any exposure to light should be allowed some access to the air, particularly when they are returned to dark storage, thus allowing density regain by air oxidation which reverses any fading incurred. The volume of air required to accomplish this is surprisingly small: for a typical 10×8 cyanotype, it is calculated that less than one cc would suffice. For this reason, archival polyester is less than ideal as sleeving for cyanotypes, because it allows the ingress of light but not of air. A preferable wrapping material would be an archival paper of a non-buffered type, eg Atlantis Silversafe Photostore; being fairly opaque but relatively porous, it will have the desirable properties of attenuating the light, but admitting the air. When on exhibition, it is obviously preferable that the mount for a cyanotype should have a deep window mat, providing a reservoir of air, which is ‘in communication’ with the ambient atmosphere. Let your cyanotypes breatheexcl;
The research described in this paper was commissioned by the National Museum of Photography, Film & Television, Bradford, UK. The author, who acts as a consultant to this museum, expresses his grateful thanks for financial support.
Buser, H. J., D. Schwarzenbach, W. Petter, and A. Ludi. 1977. The crystal structure of Prussian blue: Fe4[Fe(CN)6]3.xH2O. Inorganic Chemistry 16: 2704.
Christensen, P. A., A. Harriman, P. Neta, and M-C. Richoux. 1985. Photo-oxidation of water using Prussian blue as catalyst. Journal of the Chemical Society, Faraday Transactions I 81: 2461-66.
Crawford, W. 1979. The keepers of light. New York: Morgan and Morgan.
Herschel, Sir J. F. W. 1842. On the action of the rays of the solar spectrum on vegetable colours, and on some new photographic processes. Philosophical Transactions of the Royal Society 181–215.
Holtzman, H. 1945. Alkali resistance of the iron blues. Industrial and Engineering Chemistry 37: 855–861.
Kirby, J. 1993. Fading and colour change of Prussian blue: occurrences and early reports. National Gallery Technical Bulletin 14: 63–70.
Kissell, E. and E. Vigneau. 1999. Architectural photoreproductions: a manual for identification and care. New York: Oak Knoll Press & NY Botanical Library.
Lathrop, A. K. 1980. The provenance and preservation of architectural records. The American Archivist 43: 325–338.
McElhone, J. P. 1993. Determining responsible display conditions for photographs. Topics in Photographic Preservation 5, American Institute for Conservation, Meeting of the Photographic Materials Group, Austin: 60–72.
Moor, I. and A. Moor. 1990. Atlantis Silversafe Photostore - a suitable paper for photographic conservation. Library Conservation News 30: 4–5.
Moor, I. L., and A. H. Moor. 1997. The conservation of Anna Atkins' British Algae. Symposium on the 150th Anniversary of Photography, Vevey, Switzerland, 29 June–2 July, 1989. Vevey: European Society for the History of Photography: 86–99.
Neuzil, M. 2001. Views on the Mississippi. Minneapolis: University of Minnesota Press. Price, L. O. 2003?
Reilly, J. M. Care and identification of 19th century photographic prints. Rochester: Eastman Kodak Company: 43.
Robin, M. B. and P. Day. 1967. Mixed valence chemistry. Advances in Inorganic Chemistry and Radiochemistry 10: 294–299.
Schaaf, L. J. 1985. Sun gardens. Victorianphotograms by Anna Atkins. New York: Aperture
Schaaf, L. J. 1992. Out of the shadows: Herschel, Talbot and the invention of photography. New Haven & London: Yale University Press.
Sharpe, A. G. 1976. The chemistry of cyano complexes of the transition metals. London: Academic Press: 121–126. The Colour Index. 19??. Pigment blue 27: C.I. 77510, 77520. Society of Dyers and Colorists: 2777, 3621.
Wagner, S. S. 1991. Some recent photographic preservation activities at the Library of Congress. Topics in Photographic Preservation 4 American Institute for Conservation, Photographic Materials Group Meeting: 136–149.
Ware, M. 1994. Mechanisms of image deterioration in early photographs. London: Science Museum and National Museum of Photography, Film & Television.
Ware, M. 1999. Cyanotype: the history, science, and art of photographic printing in Prussian blue. London: Science Museum and National Museum of Photography, Film & Television.
Williams, H. E. 1948. Cyanogen compounds. London: Edward Arnold: 191–200, 226–228.
Papers Presented in Topics in Photographic Preservation, Volume Ten have not undergone a formal process of peer review.