Topics in Photographic Preservation 2009, Volume 13, Article 25 (pp. 182-197)
Presented at the 2009 PMG Winter Meeting in Tucson, Arizona
The development of a novel vapor phase iodine treatment for the reduction of silver mirroring from developed-out silver gelatin photographs is discussed. This new method was evaluated in comparison to the established treatment developed by Edith Weyde (1977) that utilizes an iodine/ethanol solution followed by fixing and bathing the photographic print. Among other benefits, the vapor treatment method removes the need for buying and maintaining anhydrous alcohol, can be performed quickly and easily, and potentially reduces risks of iodine penetration into the photograph to attack image silver.
For this study, tests were completed using identical sets of developed-out silver gelatin photographs treated using both the alcohol and vapor treatments. The samples were evaluated for reduction of mirroring, bleaching of highlights, and loss of image silver. Although further testing and analysis are underway, vapor treatment thus far has proved to be a viable option if reduction of silver mirroring is desired. A simple iodine vapor chamber is described which produces even reduction of mirroring in seconds and can be easily replicated by conservators.
The formation of silver mirroring has been investigated by a number of scientists and photograph conservators. Many agree with Hendriks (1988) who has described the deterioration to be the result of oxidation of the image silver to silver ions that can migrate through the gelatin emulsion, deposit at the surface of the photograph, and cause the characteristic reflective surface. It is likely that this effect is accelerated under high relative humidity conditions due to softening of the gelatin emulsion layer and increased mobility of the ions. Transmission electron microscopy (TEM) images have supported Hendriks’ theory by revealing small silver-containing particles surrounding a larger granule of image silver. Ulla Bøgvad Nielsen (1993) examined this theory further with TEM comparisons of freshly processed and naturally aged photograph samples, confirming that the mirroring is a result of a layer of particles on the surface of the emulsion. In addition, Hendriks (1991) discusses the evidence found by Weyde (1955) that silver ions also migrate down to the baryta (BaSO4) layer of the photograph. The migration of silver to the baryta layer is irreversible. Treatment is restricted to the accessible silver that is deposited on the surface of the print.
It is not possible to return the silver in the surface ‘mirror’ to its original location within the emulsion layer. Therefore, methods for its reduction or removal have been investigated and practiced with varying results. Luzeckyj (1999) found that removal of silver mirroring using vinyl erasers caused abrasion and burnishing of the surface. Treatment using an ethanol/water solution applied with cotton swabs was also determined to remove material from the emulsion layer of matte samples, causing voids that were visible in SEM micrographs. A third technique investigated the use of microcrystalline wax and methylcellulose to coat the surface of matte and glossy prints with silver mirroring. Luzeckyj reports that the coating was more effective for reducing the metallic sheen visible on the matte sample than that on the glossy sample. However, the surface of the matte print was altered as the coating materials filled recesses in the surface producing a smooth appearance. The only test that effectively removed the silver mirroring without altering the surface characteristics of the photograph was a wet chemical treatment using an iodine/ethanol solution (Weyde 1977) followed by fixing and bathing the print.
Literature indicates Weyde’s solution of iodine and ethanol has been effectively used for the reduction of silver mirroring on both prints and negatives. Anhydrous ethanol is necessary in order to prevent any included water from swelling the gelatin layer, thereby allowing the iodine to penetrate and attack the image silver (Hendriks 1991) in addition to the surface silver present in the mirroring. Nishimura (2001) outlines other concerns with the treatment. He states that it is difficult to monitor the photograph in the opaque deep-purple iodine solution, and treatment in a shallow bath is advocated. However, such a treatment situation could lead to increased water absorption from the air unless the bath is kept covered. In considering the ancillary benefits of this treatment, it is also important to note that based on Brandt’s (1984) study on silver image stability, iodine treatment has a potentially stabilizing effect when silver particles with adsorbed iodide ions resist oxidation by peroxides.
Once exposed to iodine in the ethanol solution the photograph is submersed in a series of fixing and washing steps to remove the silver iodide. The reaction has been described by Nishimura (2001) and outlined as follows:
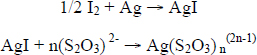
Nishimura explains that the silver thiosulfate compound is soluble in the final wash when n equals 2 or 3.
In the present study, a variation on the iodine treatment was explored. Because of its ability to sublime at room temperature, iodine could potentially be used in the gas phase to treat silver mirroring on photographic negatives and prints. In these experiments, one sample set was exposed to vapor phase iodine and compared to a second set of samples treated using Weyde’s ethanol solution of iodine. The vapor treatment successfully reduced the visual effects of silver mirroring from the surface of a naturally aged matte gelatin silver photograph during the initial tests. The paper support was also brightened. This result was expected since it is known that iodine is a mild oxidative bleach. This bleaching has been observed in the past as well during experimentation with Weyde’s formula during a PMG workshop (Nishimura 2001). Further analytical work was undertaken to assess the chemical and visual effects of both treatment approaches.
Importantly, the iodine vapor treatment removes the need for expensive anhydrous ethanol as well as reducing the risk of swelling the gelatin emulsion during the treatment step. The original hypothesis that the iodine vapor treatment only reacts with the silver particles on the mirrored surface of the emulsion was investigated using XRF, SEM, and TEM.
A total of 48 samples were removed from both freshly processed as well as naturally aged gelatin silver prints. The new gelatin silver prints were developed from a Kodak 10-step Q13 grayscale negative onto semi glossy Kodak Polymax Fine Art Paper, Figure 1.
Figure 1. Freshly processed black and white silver gelatin prints used to assess the attack of the iodine treatments on image silver. Each Dmax, Dmed, and Dmin sample was cut to provide test and control samples for each treatment.
Kodak Professional Fixer was used to process the freshly prepared photographs and in the treatment steps described below. Three sets of samples were chosen to represent a minimum (Dmin), medium (Dmed), and maximum (Dmax) image density from the sections marked 0.00, 0.70, and 1.90, respectively. An evenly exposed area was chosen for each image density sample and cut to provide both control and test sample with a shared cut edge for cross-section analysis. No silver mirroring was developed on these samples; they were used to evaluate only the effect of each treatment on image silver within the gelatin emulsion.
Naturally aged gelatin silver developed-out photographs with signs of silver mirroring and yellow colloidal silver were selected from vintage photographs of unknown age (likely 25-50 years old). The photographs were donated to the department with the understanding that sampling techniques and analysis were destructive. Control and test samples were cut in the same manner as the freshly processed photographs.
This treatment, detailed in Scheme 1, followed an adapted version of the Edith Weyde Formula. The alcohol used was anhydrous, containing 94-96% ethanol and 4-6% methanol and isopropanol. The samples tested were equilibrated prior to treatment to the laboratory conditions: approximately 70°F and 45% RH. The tray containing 0.1% (w/v) iodine in ethanol was covered with a sheet of clear acrylic during treatment to prevent moisture in the air from entering the ethanol bath.
Scheme 1.
Iodine/Ethanol Treatment
Two sample sets were tested for (A) 3 min and (B) 9 min in the iodine/ethanol solution. The length of the iodine treatment was based on a frequently reported minimum 2-3 minute treatment time as well as a maximum treatment time (9 min) that was purposefully much longer than necessary to achieve desirable results. These two treatment times were chosen to evaluate whether the aggressiveness of the iodine on image silver correlated with the exposure period.
To create a sealed vapor chamber, a glass dessicator with perforated ceramic shelf was set up with a thin layer of silicon vacuum grease spread on the lid for the seal, Figure 2. A Petri dish containing roughly ½ tsp. iodine crystals1 was placed at the bottom, below the ceramic shelf. The sealed chamber system was set into a fume hood. The crystals began to sublime and developed an even purple gas within the chamber. The chamber could be set on a warm surface to accelerate sublimation, although it was not necessary. Iodine crystals were replenished as needed.
In order to expose samples to the iodine vapor, the container was opened by sliding the lid to the side providing enough of an opening to place the samples or photographs onto the ceramic shelf. To contain some of the vapor within the chamber during this process, the fume hood exhaust was shut off briefly, samples were placed inside, the lid was replaced on the chamber, and the exhaust turned on immediately to remove any iodine gas that escaped the chamber. The concentration of gas was not monitored and may have varied somewhat between treatments. The chamber was sealed to allow the iodine to sublime for approximately 10 minutes between sample exposures.
1 Iodine is stored as a black/purple solid and sublimes at room temperature to a purple gas with an irritating odor. Iodine is CORROSIVE. It can cause eye and skin burns, severe respiratory and digestive tract irritation, and possible allergic reactions with skin. Target organs include the kidneys and thyroid. Wear protective clothing, gloves, and goggles while working with iodine in ethanol or as a gas under a fume hood. Do not inhale fumes.
Figure 2. Sealed dessicator chamber with perforated ceramic shelf for iodine vapor treatment.
Treatment of samples using the iodine vapor technique, Scheme 2, followed the steps outlined in Scheme 1 for the alcoholic iodine treatment with the exception that steps 1 through 3 were substituted with exposure of the samples in the iodine vapor chamber. Treatment times were chosen based on testing of a preliminary sample set, which indicated that (A) 30 seconds was sufficient for removal of moderate silver mirroring, and (B) 5 minutes was well over the time necessary for successful treatment of all the samples tested. Once exposed to iodine vapor, the surface of the samples obtained a purple hue. Some residues and stains on the reverse of samples also changed color in the vapor chamber. The purple hue dissipated immediately upon submersion in the fixing bath. Staining was not visible after treatment.
Scheme 2.
Iodine Vapor Treatment
A GretagMacbeth® Color Eye® XTH spectrophotometer was used in conjunction with Color iControl software to analyze the sample sets from both schemes before and after treatment. The D65 Illuminant was used with the 10° observer, spectral component included, and small area of view (SAV) attachment. This non-destructive test was used to record color measurements using the CIE L*a*b* color space. Color difference (ΔE*) was calculated by the Color iControl software. The value is calculated from changes in each L*, a*, and b* component: ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2] 1/2 where ΔL* represents the change in lightness/darkness, Δa* represents the change in the redness/greenness, and Δb* represents the change in yellowness/blueness of the sample. In addition, a 60° gloss measurement was recorded, with changes in gloss after treatment being expressed as ΔG. A-ΔG value reveals a greater gloss after treatment, and a +ΔG value indicates a more matte surface. The meter was programmed to obtain three consecutive readings and record the mean value. A calibration of the spectrophotometer was conducted at the start of each session using a standard white tile provided by the manufacturer.
Comparisons of the quantitative data with the impressions from visual inspection are essential to evaluate the reported values for ΔE*, ΔL*, Δa*, and Δb*. R. Johnston-Feller (2001) stresses that the data related to changes in color from different regions of the color spectrum are not equal to one another. It is indicated that, “one unit of color difference in the blue region does not represent the same perceived difference as one unit in the red region” (pg. 33). However, a total change in color, represented by ΔE*, is calculated based on each of the regions as equal parts in the ΔE* equation. Although a ΔE ≥ 1 is often reported as the limit for a visibly perceptible change in color, Johnston-Feller points out that this is based on an industry standard that is dependent on the equation used. It is not necessarily indicative of a just perceivable difference, and Johnston-Feller suggests the value of total color change calculated from the above equation represents approximately three times a just perceivable difference, but only under ideal viewing conditions that are rarely achieved in practice.
Results of Spectrophotometer Analysis:
Freshly Processed Samples :
Color measurements of freshly processed, non-mirrored photographs treated with the two iodine protocols were difficult to confirm visually. According to the color data, expressed in positive ΔL* values, all freshly processed samples were lighter after treatment with iodine regardless of its form. This measured change was not visibly detected despite ΔL* values as high as 2.97. Changes in Δa* (redness/greenness) were negligible with a maximum value of -0.18 reported. The changes in the yellowness/blueness were expressed in low Δb* values ranging from -0.09 (bluer) to 0.59 (yellower). There was no significant difference between the data from iodine vapor or ethanol solution treatments for the freshly processed photograph samples.
The gloss changes for all three image density sets of freshly processed, non-mirrored samples were small and seemingly random in the direction of their change. The ΔG values could not be visually confirmed; the naked eye could not detect changes between untreated controls and the iodine treated test samples.
Naturally Aged Samples:
Before treatment, Dmin samples showed no visible mirroring on the surface of the historical photograph, but the areas appeared yellowed due to either small amounts of colloidal silver or due to yellowing of the support and gelatin emulsion. Color measurements confirmed this observation as all Dmin naturally aged samples fell within the yellow area of the 1976 CIELAB color space. After both the ethanol and vapor treatments, this yellow component was reduced as evidenced by substantially negative Δb* values. Similarly, the ΔL* value indicated the samples were significantly lighter after treatment. The ΔL* values were 4.32 and 4.61 for the vapor treatment performed on two Dmin replicates, and 3.2 and 3.7 for those treated with the ethanol solution. There was negligible change in Δa* values. The large magnitude of the ΔL* and Δb* values contributed to the most significant ΔE* values calculated for any sample set tested.
Gloss values for Dmin naturally aged samples were somewhat problematic. These samples were most likely too matte to obtain reliable measurements using the 60° gloss setting available on the ColorEye XTH instrument. Typically a more oblique 85° measurement best highlights subtle changes in matte surfaces. Regardless, no change in gloss was visibly identified for the Dmin naturally aged samples.
Color data indicated that the naturally aged, heavily mirrored Dmax samples were darker after treatment, as one would expect when removing the highly reflective surface silver. Data relating to samples submersed in the ethanol treatment showed the greatest -ΔL* values. Removal of the silver mirror revealed the less reflective, rich dark image tone beneath. The Δb* values indicate a greater change in samples treated with the Weyde formula than with the iodine vapor treatment. The +Δb* values represent a greater reflectance in the yellow region of the spectrum and is attributed to the reduction of the mirroring, which often has a silvery-blue tone. The silver mirroring was observed to be more highly reflective of the shorter wavelengths of the visible spectrum. Because the silver mirror is removed by the iodine treatment, the +Δb* values are not interpreted as a change in the color of the photographic image within the emulsion, but a reduction of the density of mirror particles from the surface.
Dmed and Dmax naturally aged samples contained visible silver mirror before treatment. Both vapor and ethanol treatments were successful in reducing the mirroring, producing +ΔG (more matte) values for all samples. Changes in surface texture of the emulsion underlying the silver mirror due to treatment with either iodine method could not be confirmed through visual inspection of samples when comparing Dmed and Dmax areas.
An attempt was made to determine whether image silver within non-mirrored, freshly processed Q13 grayscale samples was affected by either iodine treatment. In addition, the XRF spectra were utilized in an attempt to identify the presence of residual iodine in the treated samples. Control and treatment samples were analyzed using an ArtTax μXRF spectrometer (Bruker). The molybdenum x-ray tube was operated at 50keV and 70μA with a helium purge gas to reduce absorption of characteristic x-rays from low atomic number elements. The area sampled was 1 mm in diameter, and data were collected during 90 second scans. Three scans were completed in the same spot on each sample, and the mean and standard deviation was found for the three analyses. Peak counts for barium (Ba), strontium (Sr), and silver (Ag) were recorded as were the counts per second (cps) for each element.
Results of XRF: Iodine & Image Silver
Peak counts for Ba vs. Ag k-alpha peaks were plotted, as were identical graphs using the counts per second. The two plots showed identical trends, therefore only peak count data were used for further assessment of the samples. A strong correlation is observed in Figure 3 between the three sample sets of varying image density; when Ag peak counts increase (e.g. in the Dmax samples), the Ba peak counts decrease. This may be the result of an attenuation effect of the barium in the sample due to screening by the overlying layer of silver. The Dmin and Dmed samples displayed very low peak counts for silver, and so the Dmax sample sets provided the best opportunity to study the potential loss of image silver due to the iodine treatments.
Figure 3. Distribution of peak count data for Ag vs. Ba collected for non-mirrored, freshly processed samples.
Figure 4 shows the mean values with error bars for Ag and Ba peak counts in the Dmax
control samples and the same samples after the two iodine treatments for both (A) short exposures and (B) long exposures. Each data point represents the average of three replicate analyses with a 3.3% error in Ag and a 1.4% error in Ba peak count data.
A small change in Ag counts is apparent when comparing the control to the two shorter treatments (liq A and vap A). The longer treatments (liq B and vap B) showed a slightly increased loss of silver content in these freshly processed, non-mirrored samples. At this scale, the proximity of the data points to one another in conjunction with the size of the error bars indicates that there is little difference between the vapor and liquid treatments with regard to their effect on image silver. Additionally, only a modest increase in image silver loss appears to occur with excessively long treatments by either method. It is recognized that these data are at best semi-quantitative and further testing and analysis using quantitative analytical techniques would be necessary to confirm any trends observed here. Importantly, visible assessment of the controls and treated samples showed no evidence of change in color or lightness for these freshly processed Q13 gray-scale samples.
Figure 4. Distribution of peak count data collected for Dmax, non-mirrored, freshly processed samples.
Cross-sectional analysis was performed for both freshly processed and naturally aged samples using scanning electron microscopy with energy dispersive x-ray spectroscopy (SEM/EDS). SEM/EDS helped to identify whether any silver iodide remained in the emulsion after treatment. SEM provided a backscattered electron (BSE) image of the samples that was then used to target specific areas for analysis using EDS, which produces an elemental analysis of the area. The technique required the removal of microsamples, which were then embedded in epoxy, ground to expose the cross section, and finely polished. Carbon coating was used to reduce charge buildup on the samples during SEM/EDS analysis.
Results of SEM/EDS: Iodine & Silver Particles
Figure 5 shows the EDS spectrum of an area of high silver content in the Dmed samples treated in the iodine vapor chamber. No iodine was present above the limit of detection for this technique, e.g. ~1%.
Figure 5. EDS spectrum of a freshly processed Dmed sample treated in the iodine vapor chamber. No residual iodine was detected.
Transmission electron microscopy (TEM) produces a beam of electrons that are accelerated and transmitted through a thin section of the original sample. The cross-section absorbs electrons depending on the composition of the material and the thickness over the section, i.e. folds appear darker as do more atomically dense features. The resulting image is magnified and displayed on a screen.
TEM analysis of control and iodine-treated sample cross sections was completed by Ann Lehman, Director of Electron Microscopy at Trinity College in Hartford, CT. Samples were embedded with Spurr’s low viscosity embedding mixture using the ‘firm standard formula’ provided by the manufacturer. Prior to embedding, samples were dehydrated using a 100% ethanol soak for 20 minutes. This was followed by a two step infiltration of 50/50 resin/ethanol for 20 minutes then 100% resin for 40 minutes. Each resin block was labeled, and then the samples were cured in a 70°C oven. The cured blocks were cut into thin sections of approximately 70 nm thicknesses using an ultramicrotome. Extensive sample preparation was necessary to produce thin cross sections in good condition for analysis. The preparation and cutting of samples was difficult as many suffered tearing and wrinkling of the photographic emulsion. Cross-sections were placed on a copper mesh support and examined using a Philips/FEI TEM at 120kV.
The TEM research is still in progress at the time of writing. Analysis of images collected by TEM will continue with more quantitative measurement of the size, distribution, and quantity of silver particles for evaluation of the effects of the different iodine treatments on silver mirroring as well as image silver.
Current Results of TEM:
TEM proved to be the most useful technique for visual analysis of cross-sections from naturally aged, heavily mirrored Dmax samples. Silver mirroring is clearly detectable at the surface of the emulsion, as shown in the before treatment image of Figure 6 (left). The silver particles at the surface often appear tightly packed and rounded in shape. Some samples exhibited gaps in the mirror layer; however, this is likely an artifact of sampling and thin section preparation. Directly below the surface of the mirroring layer lie much smaller, round silver particles that generally appear to increase in size the closer they are to the surface. Similar observations were made by di Pietro (2002) in a study of mirroring on glass plate negatives. The mirroring is clearly removed from the surface of the emulsion following iodine vapor treatment (right). Qualitative inspection of cross-sections and recorded TEM images has not yet revealed any significant changes to silver particles within the emulsion, or ‘image’ layer, following treatment. Additional, quantitative analysis is currently underway.
Figure 6. Naturally aged Dmax samples before (left) and after (right) iodine vapor treatment. The emulsion layer lies at the top of each image. The silver mirroring (left, dark layer) is largely removed following treatment (right).
In addition to evaluating the new vapor technique using freshly prepared photograph samples, two gelatin silver photographs were treated in order to gauge the effect of the treatment in actual practice. The historical photographs exhibited two varieties of silver mirroring deterioration: the print shown in Figure 7 (left), displayed a hazy silver mirror while the print shown in Figure 8 (left) was visibly mirror-like and difficult to read.
Figure 7. A matte gelatin silver print with a hazy form of silver mirror deterioration on the surface before treatment (left) and the same print after iodine vapor treatment (right). The details of the background and density of image in the hair and shadows has become more readable.
Each photograph was lightly surface cleaned to remove any dust or grime and treated for three minutes in the vapor chamber. The iodine exposures were followed by the same series of fixing and washing steps as outlined earlier in Scheme 2. Figures 7 and 8 (right) show the dramatic improvement in the appearance and readability of these historical images in specular lighting.
Figure 8. A gelatin silver print with a highly reflective form of silver mirror deterioration on the surface (left) and the same print under identical specular lighting following iodine vapor treatment (right). The details in the dense image areas have become more readable.
The iodine vapor treatment developed and tested during this research was effective in reducing silver mirror deterioration from the surface of the photograph samples tested. Color measurement, microscopy, and visual inspection confirmed this. After treatment, greater changes were clearly evident among naturally aged photographs. Naturally aged Dmin samples clearly showed the effects of what may be the mild oxidative bleaching action of iodine combined with aqueous washing, a technique known to remove soluble discoloration from fiber based materials. These slight color changes detected in the yellow/blue areas of the color spectrum should be expected as a side effect of treatment with iodine vapor as well as with the Weyde formula, especially in the highlights of silver gelatin photographs. Although further investigation is clearly necessary, there has been no significant evidence thus far to indicate that iodine vapor penetrates the emulsion and effects image silver.
These comparative XRF analyses can neither confirm nor disprove theories that iodine treatment effects image silver within the gelatin emulsion layer. However, they do suggest a potential change that is too small to identify accurately using the equipment available for this study. Further analysis of cross sections imaged using TEM will provide more information regarding the effects of iodine treatment applied to silver gelatin photographs.
The treatments applied to actual mirrored photographs helped to restore the contrast of silver densities obscured by silver mirror deterioration. Technically, image silver is lost in the treatment of these objects as the silver mirror deterioration now at the surface of the emulsion was once held within the gelatin emulsion as image silver. Once deposited on the surface, it is no longer a part of the image, but a shield, obscuring the image beneath its surface.
There are benefits and drawbacks to each treatment option. These must be thoroughly understood before considering such treatment of actual art or historical images. The chemical and appearance characterizations for samples treated by both methods, i.e. ethanol solution and vapor phase iodine treatments, was nearly indistinguishable. In addition, it was difficult to see by eye the changes measured by the analytical techniques used in this study; the exception being the clear removal of silver mirroring and a reduction of yellowness in image highlights of naturally aged photographs. This treatment has been shown to provide better stability to the photographic image after treatment, thus reducing the recurrence of mirroring (Brandt 1984). This preventative aspect should be considered in the evaluation of these techniques for conservation treatment of photographic prints and negatives.
A practical comparison of these treatment protocols revealed some benefits of using iodine vapor over an anhydrous ethanol solution. During the treatment procedure it became obvious that the samples were easier to see and monitor in the vapor chamber than in the colored iodine solution bath. In addition, since the vapor is so effective for short treatment times, it may be possible to develop a system or device for more controlled, local application of vapor. Such a treatment could avoid the ancillary bleaching effect of the iodine on image highlights if warranted. The elimination of ethanol during treatment essentially removes the risk of swelling the gelatin emulsion, eliminating one potential mode of attack on image silver. In addition, the iodine vapor treatment is considerably faster, significantly cheaper, and easier to maintain than using anhydrous ethanol baths.
As is the case with virtually all conservation treatments, it is up to the careful considerations of the conservator to decide if the benefits and potential drawbacks of the treatment are worth carrying out on the object. The data presented in this project adds to the information available to the conservator about the chemical reduction of silver mirroring.
The authors wish to thank Ann Lehman (Trinity College Electron Microscopy Facility) and Peter Bush (University of Buffalo South Campus Instrument Center) for assistance with analytical measurements. Profs. Dan Kushel, Aaron Shugar, and Judith Walsh of Buffalo State College’s Art Conservation Department are recognized for their guidance and assistance throughout this research. Henry A. DePhillips Jr. (Dept. of Chemistry, Trinity College), Doug Nishimura and Jim Reilly (Image Permanence Institute), and Mogen S. Koch (The Royal Danish Academy School of Conservation) are acknowledged for their helpful comments regarding the iodine vapor treatment. SB was supported through a Margaret & Charles Balbach Art Conservation Fellowship, a Buffalo State College Tuition Scholarship, and funding through the Leo & Karen Gutmann Foundation and the Stockman Family Foundation. GDS received research support funding through the Andrew W. Mellon Foundation.
Albright, G. E. 2004. Iodine/alcohol for reduction of silver mirroring. Unpublished notes.
Brandt, E.S. 1984. Mechanistic studies of silver image stability. 2: Iodide adsorption on silver in the presence of thiosulfate and the influence of adsorbed iodide on the catalytic properties of silver toward hydrogen peroxide. Photographic Science and Engineering. 26(1): 13-19.
Di Pietro, G. 2002. Silver mirroring on silver gelatin glass negatives. Inaugural Dissertation, University of Basel.
Eastman Kodak Company. 1985. Conservation of photographs. Kodak Publication No. f-40. Rochester, NY: Eastman Kodak Company.
Eaton, G. 1970. Preservation, deterioration, restoration of photographic images. Library Quarterly 40 (January): 85-98.
Hendriks, K., et al. 1991. Fundamentals of photographic conservation: a study guide. Toronto, Ontario, Canada: National Archives of Canada. Lugus Productions, Ltd.
Hendriks, K., and L. Ross. Jan/Feb. 1988. The Restoration of discolored black and white photographic images in chemical solutions. AIC Preprints, American Institute for Conservation 16th Annual Meeting, New Orleans. Washington, DC: AIC, 99-117.
Johnsen, J. S. 1992. Image quality of chemically restored black and white negatives. Journal of Imaging Science & Technology. 36(1) Jan/Feb.: 46-55.
Johnston-Feller, R. 2001. Color science in the examination of museum objects: non-destructive procedures. LA: Getty Trust Publications: Getty Conservation Institute, 33.
Knipe, P. 1997. The evaluation of four aqueous and non-aqueous surface-cleaning techniques on silver gelatin photographs. Topics in Photographic Preservation, vol. 7, compiled by Robin E. Siegel. Washington: American Institute for Conservation, Photographic Materials Group, 19-27.
Luzeckyj, T. and I. Brückle. 1999. Immediate and long-term effect of the treatment of silver mirroring on the surface of photographs. 695 Research Project, Buffalo State College Art Conservation Program, Buffalo, NY.
Nielsen, U. B. 1993. Silver mirror on photographs. Thesis project, Royal Danish Academy of Fine Arts, School of Conservation.
Nishimura, D. 2001. Report on the chemical treatment of photographic materials workshop: a chemist’s perspective. Topics in Photograph Preservation, vol. 9, compiled by Sarah S. Wagner. Washington: American Institute for Conservation, Photographic Materials Group, 1-43.
Reilly, J. 1986. Care and identification of 19th century photographic prints. Rochester, NY: Eastman Kodak Company.
Weyde, E. 1955. Das Copyrapid-Verfahren der Agfa [The Agfa Copyrapid Process] Mitteilungen aus den Forschungs-laboratorien der Agfa, 1: 262-266.
Weyde, E. 1977. First printed in: Handbuch der Negativ-Restaurierung, Bildarchiv Foto Marburg. Unofficial book.
Papers presented in Topics in Photographic Preservation, Volume Thirteen have not undergone a formal process of peer review.