Topics in Photographic Preservation 2011, Volume 14, Article 10 (pp. 52-58)
Presented at the PMG session of the 2010 AIC Annual Meeting in Milwaukee, Wisconsin
Abstract - Ongoing research at The Metropolitan Museum of Art in New York has focused on the possible application of anoxic environments in preventing light-fading of autochrome color screen dyes during exhibition The methodology used in the experiment was presented during the 2008 PMG Winter Meeting at the Center for Creative Photography in Tucson, AZ (Casella 2009). The current paper presents the results of the experiment. The project tested the six dyes present in the autochrome color screen. Test samples were prepared of each of the dyes. The anoxic setup was achieved by using sealed glass tubes. A group of samples was purged with argon; a second group was sealed under atmospheric oxygen conditions. The samples were exposed to light in a fading unit at the Image Permanence Institute in Rochester, NY. Spectrophotometric data was collected before and after light fading. The results show that the fading rate of all of the individual dyes is lower under anoxic conditions.
The autochrome represents the first commercially viable color photographic process, produced from 1907 to 1935 (Lavédrine 2009). It is a transparent positive on a glass support and was viewed by projection or transmitted light. Most deterioration and damage characteristics found in autochromes, such as the breakage of the support, mirroring of the silver image, glass deterioration, image layer delamination, or formation of green staining, are more or less understood (Krause 1985). However, the causes of commonly observed color shifts in the dye layer and discolorations are not entirely clear. They may have occurred during processing or over time.
Research on autochromes has been led by the French conservation scientist Bertrand Lavédrine. Light fading tests he conducted confirmed the poor light fastness of the dyes in the color screen layer. As a result, the current policy of the majority of cultural institutions, including The Metropolitan Museum of Art, has been not to display original autochrome plates, which are considered “extraordinarily light-sensitive” (Wagner et al. 2001), but to show facsimiles or simulacra.
Lavédrine identified six dyes in the color screen layer, which were used consistently during the entire production period from 1907 to 1935: Erythrosine B (C.I. 45430), Rose Bengal (C.I. 45440), Tartrazine (C.I. 19140), Patent Blue (C.I. 42051), Crystal Violet (C.I. 42555) and Setoglaucine or Flexo Blue (C.I. 42025). The dyes were mixed to form red-orange, green and blue-violet solutions with which potato starch grains were tinted to create the color screen filters.
Past research has shown that certain colorants either do not fade at all or fade less if exposed to light under anoxic conditions (Arney et. al 1979; Beltran et al. 2008; Townsend et al. 2008).
However, some colorants will fade more rapidly in the absence of oxygen (Rowe 2004), which determines the need to test each individually. At the beginning of this project, there was no existing research on the light-fading behavior under anoxic conditions of the six dyes used in the autochrome process. The first phase of research consisted of a light-fading test of each individual dye.
For each of the six dyes, four groups of samples were prepared following the historic dye concentrations and varnish recipes. The first group consisted of dyed potato starch grains encased between two varnish layers, applied to a glass support. This sample set tested the behavior of the dyes as they are found in the structure of the autochrome, without the overlaid gelatin-silver image layer. The samples in the second set consisted of dyed Whatman filter paper, a group designed to isolate the behavior of the colorants. The third group was composed of samples of the varnish layers on a glass support, allowing observation of the changes occurring in these materials alone. The final group was made up of historical plates. Spectrophotometric measurements were taken of all the samples.
An anoxia protocol was designed specifically for this project. The setup involved assembling the samples on aluminum strips, overlaid by photographic grayscales. The addition of this grayscale allowed the gradational fading rate of the dyes to be measured after light exposure. For each sample, one area was left fully exposed to light, and another fully protected from light using aluminum tape. The aluminum strips with the samples were placed inside custom-made Pyrex glass tubes, closed on each end by threaded plastic caps.
Initially, plastic caps with a rubber O-ring were used that did not provide an impermeable seal. Several attempts to use these were made that included modifications such as lining the glass thread with Teflon™ tape, or covering the gap between the glass and the cap with pressed aluminum foil. The final design used similar plastic caps that were fully lined with a thick layer of Polytetrafluoroethylene (PTFE) for a successful seal. The anoxia tubes were filled with Argon gas humidified to 45%. RP K oxygen scavengers from Mitsubishi Gas Chemical were placed inside each tube to absorb residual oxygen after sealing and Ageless Eye indicators (also from Mitsubishi GC) were included in each tube to monitor the oxygen content. These detect oxygen levels above 0.1%.
The tubes were then transported to the Image Permanence Institute in Rochester, New York, where they were placed in a light-fading unit for 54 days, generously made available to us for this project. The total light received by the samples was 8.29 Megalux-hours, the equivalent to displaying an autochrome on a light box emitting 2,000 lux for nine hours a day over a period of seven months, approximating the average time span of a two-venue museum exhibition.
After light exposure, the samples were removed from the tubes and color monitoring was repeated. Erythrosine B, which is red in appearance, and Tartrazine, which is yellow-orange, showed little change under oxygen or low-oxygen light fading. The remaining samples exposed in normal environment conditions containing 21% of oxygen, changed noticeably compared with
the samples exposed in a low-oxygen environment. The glass-supported samples show a lower degree of change in comparison with the paper samples, suggesting there is a protective role played by the overlaying varnish layer as well as the glass supports.
To quantify the degree of change, average color differences or delta E calculations were made. There is a clear reduction of the values of change for the samples exposed under anoxic conditions. However, a complete arresting of the fading was not observed.
Setoglaucine was the dye that presented the most change under low oxygen conditions, both in paper and glass-supported samples. This dye is present in the blue-violet starch filter and this degree of change is consistent with Bertrand Lavédrine’s tests that show this as being the most light-sensitive filter in the color screen.

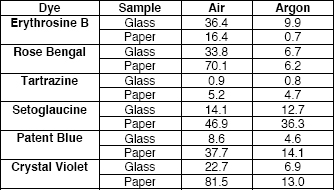
Table 1: ΔE CIE L*a*b* Values for sample areas exposed to 8.29 Mlux⋅hour
In the case of the varnish samples, the calculations for the area of full light exposure show a Delta E above 1 in oxygen, the value above which change that is discernible with the naked eye in the form of a slight yellowing; and of below 1 for the argon conditions. Inspection under long wave ultra-violet revealed a cloudy appearance on the fully exposed area of both oxygen and anoxia samples, but the degree of change observed in the sample exposed to light in normal environmental oxygen levels is much greater than that of the sample exposed under anoxic conditions.
This increase in fluorescence, as well as the yellowing of the varnish, is a result of autoxidative degradation previously studied and described by René de la Rie for varnish layers on paintings (De la Rie 1982). De La Rie noted, however, that although the degradation of dammar, which is present in both varnishes of these samples, involves photo-chemically initiated autoxidation reactions, these are followed by non-oxidative thermal processes should exposure to light continue. In this case, anoxic conditions would not be relevant.
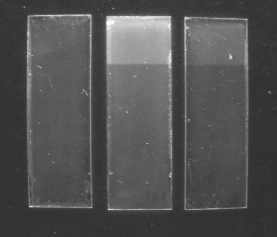
Figure 1: Varnish samples after light-aging under long wave UV. Left: control; center: air; right: low-oxygen.
Observation under the microscope shows that, when compared with the control samples, both types of exposed varnish samples present a finer cracking pattern after light exposure, a change that is equivalent between the anoxic and the oxygen environment. When looking at the transmission spectra of the varnish samples we can observe that those exposed under anoxic environment did not change significantly from the control, while the samples exposed under normal oxygen environment showed a noticeable decrease of light transmission below around 450 nm towards the ultraviolet region. This same phenomenon was observed in the glass-supported samples that showed a decrease of light transmission in this region. We can attribute these changes to the effect on varnish layer rather than a change in the dye.
In the historical samples subjected to the first fading test, the general trend observable in anoxia is a shift towards red, while in the oxygen samples the change is towards yellow/green. This is consistent with the rates of fading observed in the individual dye test. In this sample group the degree of change is reduced under anoxic conditions. Under UV illumination there was no difference between the anoxia and oxygen samples, confirming René de la Rie’s statement that after the initial oxidative degradation reactions, subsequent deterioration is independent from oxygen (De la Rie 1982).
An interesting phenomenon was observed after the fading test. The photographic density scales placed over the samples exposed under environmental oxygen conditions developed intense silver mirroring that was not observed in the samples exposed under anoxic conditions (figure 2). This is unexpected since the most extensive research on this topic by Giovanna Di Pietro (Di Pietro 2002), seemed to point to high humidity and high temperature as the main factors of this form of deterioration, independent from light exposure or presence of oxygen. In the present research the temperature and RH conditions were stable at 25 degrees Celsius and 45% RH. Contact with Di Pietro opened more possible avenues of research. However, this falls outside the main scope of the project, and no further inquiries were made.

Figure 2: Density grayscales after light-aging experiment. Left: exposed in low-oxygen; right: exposed in air.
The results of the first phase of tests suggested that there could be benefits for these dyes from using anoxic conditions during display. The limited exhibition of original autochrome plates could therefore be possible under controlled conditions. A second light fading test of the dyes in their red-orange, green and blue-violet combinations was carried out in 2009-2010 using the same methodology of the first test. These results will be published in ICOM-CC’s 16th Triennial Conference preprints in September 2011.
The possibility of exhibiting autochromes meant that an anoxic sealed package had to be designed and exhibition light sources researched. Primary goals for the package were: a passive system, straightforward in application, and economically viable. The initial enclosure design was modeled on the one used by Ralph Wiegandt for daguerreotypes. This utilizes a silicone gasket held under pressure. For our purposes we modified the package to have glass on the front and back for transmitted-light viewing of the object, and added scavengers within the enclosure to absorb any residual oxygen.
A fragment of a historical plate and three red-orange, green and blue-violet samples were bound together, and matted. The mat verso had a cutout to insert oxygen scavengers and an indicator. Aluminum foil was applied to the edges of the mat to protect it from the silicone grease used on the package. The mat was placed between two glasses with a silicone gasket. The gasket was cast with platinum-catalyzed silicone P-4 from Silicones, Inc. (www.silicones-inc.com) that passed the Photographic Activity Test, the Oddy test, and AD strip acid detection in tests carried out by Ralph Wiegandt at George Eastman House (Wiegandt, personal communication). To improve the contact of the gasket with the glass, silicone vacuum grease was applied along the edge. The thickness of the gasket was slightly deeper than that of the matted object. The package was assembled inside a glove bag that had been purged with argon gas humidified to 45%. Metal binder clips maintained an even pressure on the gasket and the glazing, providing the seal. Unfortunately, the glass on this package completely shattered after three days, possibly due to physical expansion of the scavengers. Modifications to this package were tested. An improved design is described in the accompanying article (Casella, Sanderson, 2011).
Acceptable light sources for possible exhibition of autochromes should emit no UV, no heat, and provide good white balance. Initially it was thought that low energy emission would also be required. This, however, proved not to allow for optimal rendering of the autochrome image.
New light sources recently on the market in 2009 appeared to have great potential in complying with these prerequisites. LEC or Light Emitting Capacitor flat panels emit low energy, no UV and no heat. A unit by the company E-Lite was tested. LEC panels used to be bluish/green but this has been corrected by adding reddish colorants to the panel, and the light output has a more neutral tone. The panel proved not to be bright enough for rendering the details of the autochrome.
LED or Light Emitting Diodes are small light bulbs which produce a very bright light. An LED light panel by Rosco was tested. The color tone and output was very satisfactory but it was found to generate excessive heat, reaching temperatures around 30 degrees Celsius or 86 Fahrenheit after a six hours, measured with a mercury thermometer in direct contact with the panel. The output of energy necessary for a good rendering of the image was 2,000 footcandles in direct contact with the panel. This value reduced to 200 footcandles if the autochrome plate was at a distance of three feet from the light source. A possible design for a workable display case might place the light source at sufficient distance from the object and include a cooling system or incorporate good ventilation.
OLEDs or Organic LEDs have just started to appear in the market and appear to have promising potential but were not available for testing in North America in 2009.
Although it is clear that keeping autochromes protected from light and at cold temperatures will preserve them indefinitely, this experiment has confirmed that low-oxygen environments reduce the fading rate of the dyes present in the color screen. Although low-oxygen does not eliminate fading, it significantly reduces it within a limited amount of light exposure, making exhibition under controlled conditions a viable option. The dye Setoglaucine appears to develop overall yellowing at all levels of light exposure. A second experiment was concluded in 2010 to establish the exact levels of light after which there is noticeable change for the autochrome dyes in their red-orange, green and blue-violet combination the results of which will be presented and published at the 16th ICOM-CC Triennial Conference in Lisbon, Portugal.
This research was carried out during a Research Scholarship by the author at The Metropolitan Museum of Art. It was made possible thanks to the endorsement of the Andrew W. Mellon Foundation and The Metropolitan Museum of Art, especially the Departments of Photographs and Scientific Research. The author is deeply grateful for the help and supervision of Nora Kennedy, and for the support of Masahiko Tsukada, Bertrand Lavédrine, the Image Permanence Institute (especially James Reilly, Andrea Venosa, Doug Nishimura and Dan Burge), Malcolm Daniel, Marcie Karp, Marco Leona, Eileen Travell, Lisa Barro, Barbara Brown, Guida Casella, Silvia Centeno, Tak Izawa at Mitsubishi Gas Chemical America, Mark Jacobs, Angeletta Leggio, Alejandra Mendoza, Hanako Murata, Sylvie Pénichon, Adriana Rizzo, Katie Sanderson, Nobuko Shibayama, John Tagg, Yae Takahashi, Gawain Weaver and Ralph Wiegandt.
LUISA CASELLA
Photograph Conservator, Harry Ransom Center – The University of Texas at Austin
Papers presented in Topics in Photographic Preservation, Volume Fourteen have not undergone a formal process of peer review.