
Topics in Photographic Preservation 2011, Volume 14, Article 28 (pp. 168-185)
Presented at the 2011 PMG Winter Meeting in Ottawa, Canada
This technical study was conducted during the author’s second year in the Winterthur/University of Delaware Program in Art Conservation (WUDPAC). Three whole-plate hand-painted tintypes were examined and analyzed in order to gain a better understanding of the materials used in their manufacture. Two of these tintypes belong to the Smithsonian National Museum of American History’s (NMAH) Photographic History Collection and one tintype is from the WUDPAC study collection. The selected tintypes are heavily over-painted portraits of women which share several similar painted elements including an opaque purplish background, white lace, gold jewelry, pink lips, and dark hair. This study includes a literature review as well as the visual examination and scientific analysis of the chosen tintypes. The following analytical techniques were employed: ultraviolet induced visible fluorescence, x-ray fluorescence (XRF), Fourier-transform infrared spectroscopy (FTIR), Raman spectroscopy, scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS), gas chromatography-mass spectrometry (GC-MS), cross-section microscopy, and polarized light microscopy (PLM). The technical analysis concentrated on the hand-painted elements with a particular focus on the background paint. An oil binder was identified as well as several pigments including lead white, calcium carbonate, vermilion, chrome yellow, umber, carbon black, and Prussian blue. Analytical results were compared with descriptions of hand-painting techniques and materials found in several 19th-century photographic manuals. The materials identified with this technical study appear to correspond with published hand-coloring recipes. Additional research is needed to identify possible trends in application techniques and materials used.
The reproduction of true color was a major challenge to 19th-century photography. Soon after the 1839 announcement of the daguerreotype, several photographers began experimenting with techniques which they hoped would produce color photographs. However, it took several decades before the first commercially successful color process, the autochrome, was announced in 1907. In order to compensate for their monochromatic nature many 19th-century photographs were hand-painted with dry pigments, watercolors, oil paints, crayons, or pastels.
Painted photographs held an uncomfortable position relegated between art and photography. As Alfred H. Wall explains in his 1861 treatise A manual of artistic coloring as applied to photographs; “the artist curls his lip at them, because, as he says, they are not paintings; and the photographer sneers at them, because, as he says, they are not photographs” (2). Many viewed the coloring of photographs as inherently false and demeaning. As a result hand-colored photographs were often excluded from salons. In contrast, the public desired colored portraits because they were more flattering and lively compared with those created by photography alone. For this reason, the coloring of commercial portraiture was immensely popular and considered appropriate. In his 1858 photographic manual Auguste Belloc summarized the situation as follows; “coloring is inexcusable except to portraitists responding to the demands of the public” (Swan 1989, 151).
By the 1850s hand-coloring kits were widely available for purchase from photographic suppliers. These kits were purchased complete with camel’s hair brushes, mixing saucers, binders, and dry pigments. The bottles of pigment were often labeled with names indicating the areas for their use; for example “ladies flesh color” (Ferguson 2008, 17). Several 19th-century photographic manuals give explicit instructions regarding the choice of pigments to be used for specific areas of a portrait. This seems to suggest that the hand-coloring of 19th-century photographs was rather formulaic.
Tintypes were among the photographic processes most frequently and most extensively hand-colored in America (Burns 1995, 1). Customers could order their tintypes either lightly retouched or completely covered with a heavy application of paint. Of those which were heavily painted, the whole plate size (6.5 x 8.5 inches) or larger was most common. Whole-plate hand-painted tintype portraits became particularly popular in America after the Civil War (Trask [1872] 1973, 8). These portraits were usually priced by how much time it took to color them, so large heavily painted plates were the most expensive (Burns 1995, 56). Consequently, these tintypes were considered precious and were often framed and hung in the home in the manner of a traditional painting (Burns 1995, 7).
Tintypes are unique objects which could not be duplicated through contact printing, due to the opaque metal support, or solar enlarging, due to the collodion process. Therefore, large plate tintypes could only be made by original creation in a camera or by re-photographing an existing image. In some instances, clients went to a photographer specifically for the production of a whole-plate hand-colored tintype. However, it was more common that this type of portrait was created by re-photographing a smaller image. Hired agents, who functioned as traveling salesmen, went door-to-door and gathered photographs, of any format, from clients. The agent would then mail this to the proprietor of the company to be re-photographed into a large tintype plate, hand-colored, and returned (Burns 1995, 39). In his book Forgotten marriage: The painted tintype & the decorative frame 1860-1910, Dr. Stanley Burns (1995), a photographic historian and collector, identifies and describes the working methods of several copying companies, including The New England Copying co., Ferro Chromo, North America Portrait co., and Electrograph Portraits.
This technical study was conducted during the author’s second year in the Winterthur/University of Delaware Program in Art Conservation (WUDPAC). The project fulfilled requirements for the Advanced Analytical Techniques course taught by Dr. Joseph N. Weber and Dr. Jennifer L. Mass. In this course students are introduced to the theory and practical application of instrumental analytical techniques commonly employed in conservation science. The purpose of this project was to provide the author with an opportunity to gain skill and experience working with a variety of instrumental analytical techniques. In addition, the goal of this technical study was to characterize the materials and gain familiarity with the methods used to create the whole-plate hand-painted tintypes selected for study. It was hoped that this technical study would reveal trends in selection and/or application of pigments, binders, and varnishes applied to the tintypes. In order to do this, the analytical data obtained from the study was compared with information gathered from 19th-century photographic manuals.
The technical study began with a literature review on the subject. This was followed with a brief visual examination of one whole-plate hand-painted tintype from the WUDPAC study collection as well as approximately 25 hand-painted tintypes from the Photographic History Collection of the Smithsonian National Museum of American History (NMAH). Six whole-plate hand-painted tintypes from the NMAH collection were chosen based on their similarities with the WUDPAC tintype including opaque purplish backgrounds, white lace, gold jewelry, flesh tones, and dark hair. These six tintypes were loaned to Winterthur Museum for their inclusion in the technical study.
None of the tintypes included in this technical study have known creation dates. Tintypes were popular from approximately 1853 to 1930. Whole-plate hand-painted tintype portraits were particularly popular in America after the Civil War (Trask [1872] 1973, 8). However, it is possible that these tintypes are copy plates which were created by re-photographing the original image at a later date. In fact, two of the NMAH tintypes (Figs. 6 and 7) clearly reveal the edge of the original photographs as well as the copy stand mechanism used during the creation of these copy plates.
Three whole-plate hand-painted tintype portraits of women were analyzed. Emphasis was placed on the hand-painting media (pigments and binders) and varnish layers. The chosen plates share several similar painted elements and these areas became points of concentration and comparison for the analysis. One of the tintypes (Fig. 1) belongs to the WUDPAC study collection. The other two tintypes (Fig. 2 and 3) are part of the NMAH Photographic History Collection. All three tintypes were examined under normal light, ultraviolet light, and with stereo-binocular magnification. Further analysis of the two NMAH tintypes was limited to non-destructive techniques including; x-ray fluorescence (XRF) and Raman spectroscopy. Limited sampling of the WUDPAC tintype was permitted. Thus, in addition to the aforementioned non-destructive techniques, samples were taken for analysis with Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS), gas chromatography-mass spectrometry (GC-MS), cross-section microscopy, and polarized light microscopy (PLM).

Fig. 1: WUDPAC

Fig. 2: NMAH (75.17.957.2)

Fig. 3: NMAH (6912A)
Four additional whole-plate hand-painted tintype portraits from the NMAH collection (Figs. 4-7) were also examined under normal light, ultraviolet light and stereo-binocular magnification. These tintypes were not analyzed with any other techniques.

Fig. 4: NMAH (6912B)

Fig. 5: NMAH (86.538.1)

Fig. 6: NMAH (75.7.27)

Fig. 7: NMAH (78.31)
The literature which specifically addresses the hand-coloring of tintypes is fairly limited. There are several 19th-century sources that are rich in information about the production of tintypes but either omit or minimally refer to their hand-coloring (Estabrooke [1872] 1972; Estabrooke 1878; Towler [1864] 1969; Trask [1872] 1973). On the other hand, there are several 19th-century photography manuals which are devoted to the practice of hand-coloring, but these focus on paper prints with minimal or no reference to tintypes (Ayres 1878; Hall [1861] 2009). Several 20th-century sources have focused on the historical significance of the tintype (Kashner 2008; Rinhart 1999; Schimmelman 2007). However, only two recent sources have provided much insight into those that are hand-painted (Henisch 1996; Burns 1995). Henisch (1996) devotes a chapter of his book The painted photograph: 1839-1914 to the tintype, and Burns’ (1995) Forgotten marriage: The painted tintype & the decorative frame, 1860-1910 is likely the most comprehensive source on the subject available to date. Both sources contain numerous historical references, a plethora of images, and a great deal of insightful research. However, neither presents analytical data or technical research into the materials and techniques used in the production of these objects.
The limited literature available which specifically addresses the hand-coloring of tintypes suggests that many variations of media could be used. The use of heavier oil-based paints predominated (Henisch 1996, 104), but watercolor and gouache were sometimes used for details in the face and hands, because it allowed the photographic details to show through (Burns 1995, 57). Tintypes were also sometimes colored with crayon or pastel and sealed under a sheet of glass (Burns 1995, 40). India ink, “chemical coloring”, and electrolytic processes are also believed to have been used (Burns 1995, 40-42).
Several 19th-century photographic manuals were consulted during the literature review portion of this study. Many of these manuals give explicit instructions regarding the choice of materials to be used in specific areas of a portrait. For example, in a section titled ‘Painting the lips’, George Ayres instructs the reader to;
“use Indian red with a little crimson lake for the upper lip, for the lower lip wash it first with thin vermilion, or orange chrome and rose madder, and in either case model and shade it afterwards by stippling with pink madder” (72)
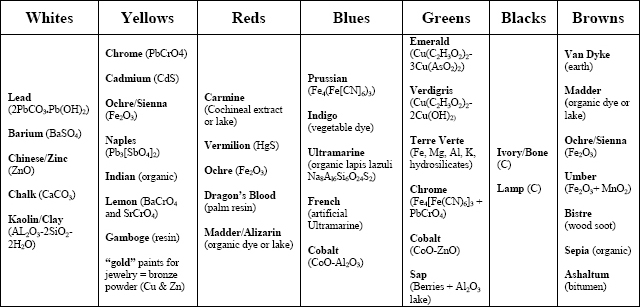
This seems to suggest that, if the colorist performed his work ‘by the book’, then the hand-coloring of photographs could be rather formulaic. However, practically every manual supplies slightly different instructions. Table 1 shows a compilation of all of the pigments and dyes suggested in all of the manuals consulted during this study. Nearly every pigment and dye available in the late 19th-century is mentioned. So obviously, a creative colorist could have produced a portrait using an incredibly unique mixture of pigments if he wished.
Table 1: Suggestions for pigments and dyes to be used in the hand-painting of photographs (Compiled from 19th-century photographic manuals)
The initial examination of approximately 25 hand-painted tintypes pulled from the NMAH collection revealed that the opaque purplish background, seen on all of the plates chosen for this study, appears to be quite common. Several images of tintypes with comparable backgrounds were also discovered during the literature review. Dr. Stanley Burns, a collector and respected scholar of hand-painted tintypes, was contacted via email and he agreed that this background style was widely popular. However, there is surprisingly little mention of these backgrounds in the literature and the few sources which were found are not specific to tintypes.
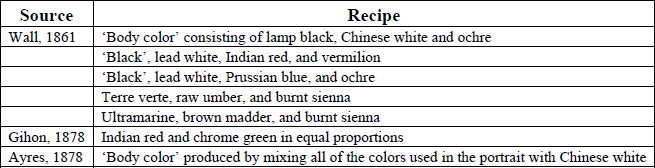
In a section entitled “Opaque Backgrounds” from How to paint photographs in watercolors and in oil by George Ayres (1878) the following is stated:
“Although it is the practice of some artists to meet the difficulties arising from very dark or otherwise objectionable backgrounds in the photograph by painting them entirely in ‘body color’ (mixing all the colors used with Chinese White) the practice is at best considered inartistic and open to many objections” (110).
John Gihon similarly explains the use of the dark opaque backgrounds in his 1878 Photographic colorists guide:
“99 out of 100 photographs that are furnished to the artists for coloring purposes have originally abominable backgrounds. The greater part of them is disfigured by mechanical defects…impossible scenery…scenic effects…The best thing that the artist can do is to blot all of these discrepancies from his picture as soon as possible” (42)
One section of A manual of artistic coloring as applied to photographs (Wall 1861) may also refer to this style of background.
“The figure should always stand prominently before the background, which should consequently be unobtrusive and retiring, with but few details, and no pure colours; dark backgrounds give the most forcible effect…To prevent any appearance of the figure being inlaid, Sir Joshua Reynolds says the ground must partake of the colour of the figure or contain such a mixture as to exhibit in it something of every colour in the palette” (36).
These sources suggest that the dark opaque neutral backgrounds found on many hand-painted tintypes are the result of the colorists’ attempt to conceal the distracting original photographic backgrounds. Several 19th-century manuals also suggest that it was common practice to create these background paints by mixing every color used on the palette during the coloring of the rest of the portrait. However, a wide variety of other recipes were also found for the creation of neutral opaque backgrounds in the manuals. Table 2 includes a list of several recipes for neutral opaque backgrounds identified through the literature search.
Table 2: Suggestions for neutral opaque backgrounds (compiled from 19th-century manuals)
Since this background appeared to be ubiquitous, it became a central focus of the research. It was of special interest to discover what materials the background paints were made from and if there were any trends in their production.
Examination, sampling, analysis, and interpretation of data were carried out in the Paper Conservation Lab and the Scientific Research and Analytical Laboratory (SRAL) at Winterthur Museum. The procedures and instrumentation used in this study are described below.
Stereo-binocular Magnification
All seven tintypes were examined using a Leica MS5 stereo-binocular microscope with magnification capability of 6-40X. Digital images were captured with a Nikon DXM 1200F digital camera in conjunction with Nikon ACT-1 software version 2.63.
Ultraviolet (UV) Induced Visible Fluorescence
All seven tintypes were also examined under UV illumination using a Mineral Light® Lamp (Model UVGL-58) with 254 nm (short-wave) and 366 nm (long-wave) capability. Any fluorescence was noted. In addition, each tintype was photographed under UV fluorescence using a Nikon D200 digital SLR camera equipped with a Wratten UV and 40 red filter.
Energy-dispersive X-ray Fluorescence Spectroscopy (ED-XRF)
ED-XRF spectroscopy was used in a qualitative fashion on the three tintypes chosen for analysis. Areas of the purplish background, gold jewelry, white lace, pink lips, and dark hair were analyzed in-situ on each plate in order to determine the elemental composition of the sample area. This information was helpful in determining possible pigments used. An ArtTAX μXRF spectrometer equipped with a molybdenum tube was used at a current of 600μA, a voltage of 50keV, and a collection time of 100 seconds in air. The spot size of analysis was approximately 70-100 microns and the element detection range was from potassium (K) to uranium (U). To collect data, the tintype was placed on an Ethafoam support near the incident beam. The beam was then precisely positioned using controls accessed through Intax software (version 4.5.18.1). An integrated CCD camera allowed for a magnified image of the region of analysis to be acquired. The spectra were interpreted using the Intax software.
Raman Spectroscopy
The areas of the three tintypes previously examined with XRF spectroscopy were also analyzed with the Renishaw Invia Raman spectrometer. The spectrometer is equipped with a microscope and a stage measuring 3 x 15 x 4.5 inches. The areas were analyzed in-situ by placing the tintypes directly onto the microscope stage. A 785nm diode laser was used for data collection. The area of analysis measured approximately 1μm by 20μm. An extended scan from 100-2200cm-1 was collected using WiRE 2 software. Spectra were collected using a range of laser powers (0.1-5%), scans (1-4), and times (10-30 seconds). Published Raman spectroscopic libraries, including the University of College London’s online library (2009), were used for reference spectra.
Scanning Electron Microscopy with Energy-dispersive Spectroscopy (SEM-EDS)
A sample of the lacquer, collodion binder, and paint layers was removed from an area of previous damage to the background of the WUDPAC tintype. A new #11 scalpel blade was used under magnification to remove the sample. The sample was immediately transferred to a carbon stub covered with a double-sided carbon tape (to reduce charge build-up) and placed on its side so that its cross-section could be analyzed. A Nikon SMZ800 microscope and a Sony Trinitron monitor was used during the removal and preparation of the sample. The Topcon ABT-60 scanning electron microscope was used with an accelerating voltage of 20kV, a stage height of 20mm, and a sample tilt of 20°. The EDS data was produced with a Bruker X-flash detector and microanalysis Quantax model 200. Esprit 1.8 software was used to interpret spectra and produce false-color elemental maps of the sample.
Fourier Transform Infrared Spectroscopy (FTIR)
The same sample that had been removed from the background paint of the WUDPAC tintype and analyzed using SEM-EDS was also used for FTIR transmission mode analysis. The sample was removed from the carbon stub used for SEM and placed onto a diamond half cell. The sample was then rolled flat with a steel micro-roller in order to decrease its thickness and increase its transparency. A Thermo Scientific Nicolet 6700 FT-IR spectrometer with a Nicolet Continuμm FT-IR microscope was used to acquire data. For each analysis 128 scans from 4000 to 650cm-1 were obtained and averaged at a spectral resolution of 4cm-1. Spectra were collected and analyzed using Omnic 8.0 software, then compared with reference spectra in commercial reference spectral libraries, including the Infrared Raman Users Group (IRUG).
Gas Chromatography-Mass Spectrometry (GC-MS)
In order to gain information about the lacquer, varnish, and paint binder a sample was removed from the background of the WUDPAC tintype. This sample was removed from an area of previous damage and contained the lacquer, collodion binder, and paint layers. A new #11 scalpel blade was used under magnification to remove the sample. The sample was placed into a capped heavy-walled glass vial and given to Dr. Chris Peterson (Consulting Scientist, Winterthur Museum) for GC-MS analysis. To reduce the molecular weight and make the components more volatile, the sample was derivitized by adding 100μL of 1:2 MethPrep II reagent (Alltech) in benzene to the vial. This converts carboxylic acids and esters to their methyl ester derivatives. The vial was warmed at 60°C for one hour in the heating block, then removed from the heat and allowed to cool to room temperature. A Hewlett Packard (HP) 6890 series GC system equipped with a HP5973 mass selective detector, a HP7683 automatic liquid injector, and a HP59864B ionization gauge controller was used for the analysis. The Agilent Technologies MSD ChemStation control software was used along with the Winterthur RTLMPREP method with conditions as follows. The inlet temperature and the transfer line temperature to the MSD (SCAN mode) was set to 300°C. A sample volume (splitless) of 1µL was injected onto a 30m×250µm×0.25µm film thickness HP-5MS column (5% phenyl methyl siloxane at a flow rate of 1.5 mL/minute). The oven temperature was held at 50°C for two minutes, then programmed to increase at 10°C/minute to 325°C where it was held for 10.5 minutes for a total run time of 40 minutes.
Cross-Section Microscopy
A sample containing the lacquer, collodion binder, and paint layers was removed from an area of previous damage to the background of the WUDPAC tintype. A new # 11 scalpel blade was used under magnification to remove the sample. This sample was then cast into a cube of Extec clear polyester resin (a methyl methacrylate monomer polymerized with a methyl ethyl ketone catalyst). The sample was allowed to cure under a tungsten halogen bulb for approximately 24 hours. Once hardened, the sample was progressively dry-polished to reveal the cross-section using Micro-Mesh silica-embedded polishing cloths with grits ranging from 1,500 to 12,000. The cube was then mounted for viewing onto a glass slide using putty and cover-slipped with petroleum benzine. The sample was examined using a Nikon Eclipse 80i Binocular Microscope with 4x, 10x, and 20x objectives, 10x oculars, and a Nikon Excite 120 Mercury Lamp for reflected visible light and reflected ultraviolet light. Digital images were captured with a Nikon Digital Eclipse DXM 1200F camera in conjunction with Nikon ACT-1 software version 2.63.This microscope is also equipped with the following filter cubes: BV-2A (Excitation 400-440 nm, Barrier 470 nm, Dichroic mirror 455 nm), B-2A (Excitation 450-490 nm, Barrier 520 nm, Dichroic mirror 505 nm), and G-1B (Excitation 546/10, Barrier 590 nm, Dichroic mirror 575 nm). The following fluorochrome stains were also used to characterize the binding media
Polarized Light Microscopy (PLM)
A scraping of the background paint layer was removed from an area of previous damage to the WUDPAC tintype. A new #11 scalpel blade was used under magnification to remove the sample. The sample was mounted onto a glass slide using Cargille Meltmount (refractive index of 1.66) and analyzed using a Nikon Labophot 2-POL polarizing light microscope with a transmitted visible light source. This microscope is equipped with 10x, 20x, and 40x objectives, and 10x oculars. The pigments’ characteristics were noted and compared to known pigments from the examiner’s personal reference set as well as information in Painting materials: A short encyclopedia by Gettens and Stout (1942) and the Pigment compendium (Eastaugh 2008).
Examination under normal light and stereo-binocular magnification revealed several clues about the application of the background paint. In all seven tintypes, the backgrounds appear to have been applied after the figures were outlined. The background paint on three of the tintypes also has a textured appearance indicating that the paint was applied with a ‘sponging’ technique. In all seven tintypes, the background paint is also matte and unvarnished.
When viewed under long-wave UV illumination, it was discovered that the WUDPAC tintype had a varnish coating with a bright orange autofluorescence characteristic of shellac. This varnish is selectively applied over the woman’s dress, hair, and areas of her face. When viewed under normal light these areas are noticeably glossier. Four of the NMAH tintypes had a varnish coating with a yellow-green autofluorescence characteristic of a natural resin. This varnish layer was visible in all areas except the background. The white lace elements of the WUDPAC, NMAH (6912A), and NMAH (6912B) tintypes fluoresced bright white suggesting an oil paint with high proportions of lead white (Buck, 2010). The white lace of NMAH (75.17.957.2) and NMAH (78.31) had a bright yellow autofluorescence suggesting the presence of zinc white (Simpson-Grant 2000).
A summary of the data obtained from XRF and Raman analysis is contained in Tables 3 and 4.
Table 3: Data obtained from XRF analysis (Peaks listed roughly in order from largest to smallest)
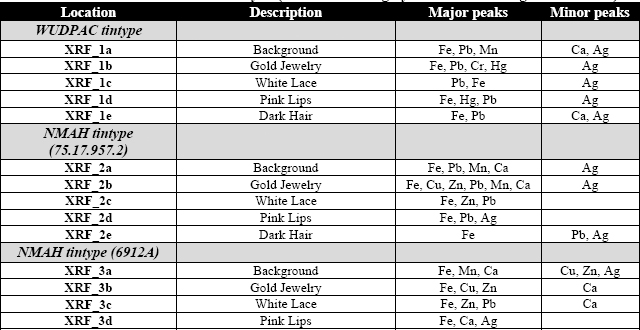
The XRF data provided elemental identification which could be used to distinguish a list of possible inorganic pigments used. There was a large iron (Fe) peak seen in all spectra. In addition, minor silver (Ag) peaks were present in many areas. These peaks are likely due to the iron support and the silver image material. The presence of lead (Pb), mercury (Hg), chromium (Cr), copper (Cu), zinc (Zn), manganese (Mn), and calcium (Ca) are likely contributed from hand-painting materials. In several cases Raman analysis provided additional information which could be used to identify specific pigments used. All of the sites analyzed with XRF were also analyzed with Raman; however a high amount of fluorescence in many areas saturated the detector making it impossible to obtain useful data.
Table 4: Data obtained from Raman analysis
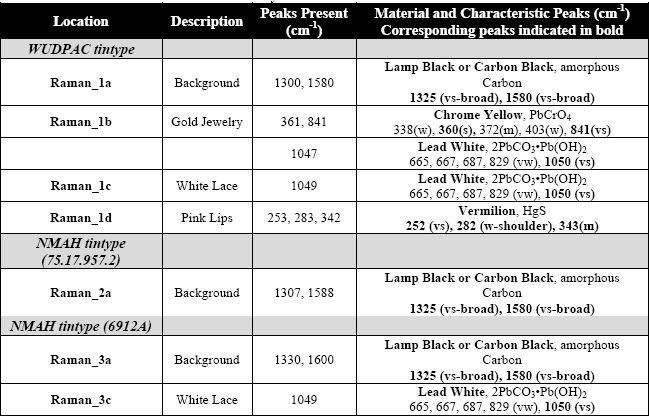
The Raman spectra obtained from the backgrounds of all three tintypes had broad peaks near 1325 and 1580 cm-1. This is characteristic of amorphous Carbon (carbon black). The spectrum obtained from the gold jewelry of the WUDPAC tintype exhibited strong peaks near 360 and 841 cm-1 indicating the presence of chrome yellow. This spectrum, as well as the spectra obtained from the white lace of both the WUDPAC tintype and the NMAH (6912A) tintype contained a strong peak at 1050 cm-1, indicating the presence of lead white. The spectrum obtained from the pink lips of the WUDPAC tintype (Fig. 8) has peaks at 253, 283, and 342 cm-1 indicating the presence of vermilion.
FTIR analysis was performed on a sample taken from an area containing the background paint of the WUDPAC tintype. The spectrum obtained (Fig. 9) showed peaks at 2930 cm-1 and 2856 cm-1 (-CH stretching) as well as 1712 cm-1 (C=O stretching). This likely indicates the presence of a drying oil and/or shellac. A peak at 2096 cm-1 (C≡N) is definitive for the presence of Prussian blue.
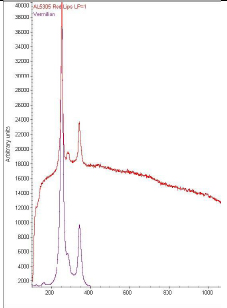
Figure 8: Raman spectrum obtained from pink lips of WUDPAC tintype – Raman_1d (top) with reference spectrum for vermilion (bottom)
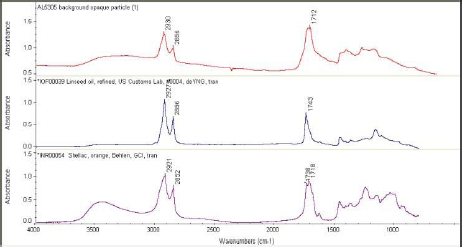
Figure 9: FTIR spectrum obtained from background paint of WUDPAC tintype FTIR_1a (top) with reference spectrum for linseed oil (middle) and shellac (bottom)
Cross-section microscopy and SEM-EDS analysis was performed on samples from an area containing the background paint of the WUDPAC tintype. This analysis revealed useful information regarding the stratigraphy and the elemental composition of the samples. SEM-EDS elemental mapping identified lead (Pb), iron (Fe), calcium (Ca), silver (Ag), manganese (Mn), aluminum (Al), silicon (Si), and carbon (C) distributed throughout the sample. Table 5 contains a summary of the data collected.
Table 5: Data obtained from SEM-EDS of sample from background area of WUDPAC tintype
When the cross-section sample was viewed under the BV-2A (UV) filter it was discovered that the lacquer layer had a bright orange autofluorescence characteristic of shellac. In addition, the paint layer exhibited a positive reaction for lipids (oils) when stained with Rhodamine B (RHOB). Fluorescein Isothiocyanate (FITC) and Triphenyl Tetrazolium Chloride (TTC) were also applied but no reactions were observed, which suggests there is little to no protein or carbohydrate present.
A sample from an area containing the background paint of the WUDPAC tintype was also analyzed with GC-MS. The chromatogram contains peaks at retention times of 16.33, 18.84, and approximately 20.7 minutes. These peaks correspond to azelaic acid (dimethyl ester), palmitic acid (methyl ester), and stearic acid (methyl ester), respectively. This indicates the presence of a drying oil.
PLM was used to analyze a sample taken from the background paint of the WUDPAC tintype. Chalk, lead white, carbon black, a brown iron oxide, and a red lake were identified based on their color, shape, refractive index, and birefringence.
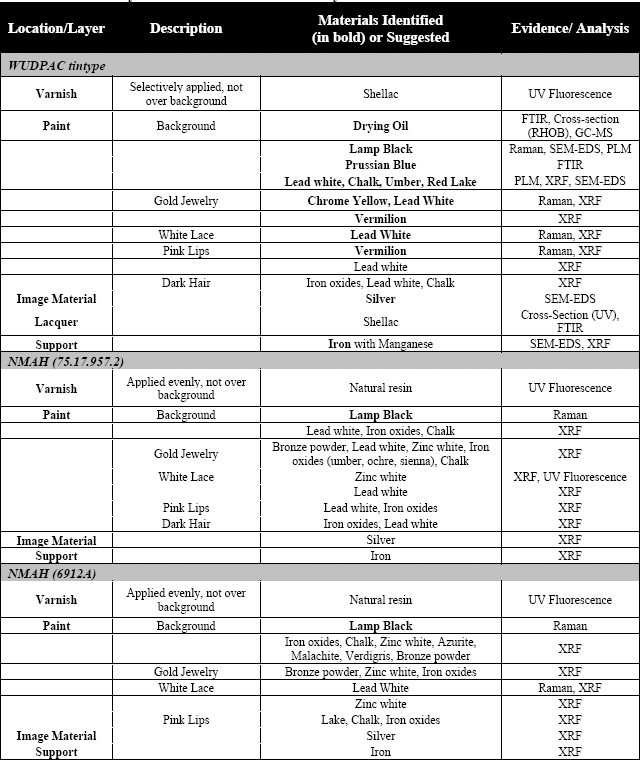
Table 7: Summary of conclusions based on analytical results
Varnish
UV induced fluorescence was helpful in the characterization of the varnish coatings. The WUDPAC tintype had a coating with a bright orange autofluorescence characteristic of shellac. The coating was applied selectively over the woman’s dress, hair, and face but not over the background. This absence of the shellac varnish coating in the background areas was confirmed through cross-section microscopy using reflected UV light. FTIR and GC-MS analysis of a sample also taken from an area of the background paint did not detect the presence of shellac or any other resin. This suggests that the background of the WUDPAC tintype was painted without pre-varnishing.
The NMAH tintypes appeared to have a varnish coating that was applied evenly to all areas except the background. The yellow-green autofluorescence of the coatings is suggestive of a natural resin varnish. Sampling and analysis with FTIR or GC-MS would be necessary to support this suggestion. In addition, cross-section microscopy of a sample from the backgrounds would be helpful in determining if this area was painted over a varnish layer or directly over the collodion image layer. There are no visible signs that the varnish was applied after the background paint.
Background
Cross-section microscopy with fluorochrome staining, FTIR, and GC-MS analysis of samples from the WUDPAC background paint corroborate the presence of an oil binder. Raman spectroscopy showed that the background paints on all three tintypes contain amorphous carbon, likely in the form of a carbon black pigment. PLM of the WUDPAC tintype confirmed the presence of a carbon pigment, likely lamp black. PLM of the WUDPAC tintype also showed the presence of an iron oxide pigment. SEM-EDS analysis of this area indicated iron and manganese content suggesting that umber is present. XRF of the backgrounds on both of the NMAH tintypes also show the presence of iron and manganese indicating that umber or another iron oxide may be present. However the iron tintype support contributes to these peaks as well. The only way to be sure that iron is also present in the paint layer would be to do SEM-EDS mapping or remove a sample and perform PLM or XRF on the paint layer alone, which was not permitted for this study.
PLM of the WUDPAC tintype indicated that chalk and lead white are also present in the background paint. XRF analysis suggests that both NMAH tintypes may also contain chalk, likely used as a filler. Lead was also detected by XRF in NMAH (75.17.957.2) indicating that lead white may be present. FTIR analysis indentified Prussian blue in the WUDPAC background paint. PLM also revealed the presence of a red lake in the background of the WUDPAC tintype. The detection of aluminum (via SEM-EDS) may be due to the presence of the red lake as these are made by precipitating an organic dye onto an inert inorganic substrate, typically aluminum hydrate (Gettens 1942, 124). XRF analysis of NMAH (6912A) revealed minor peaks for copper and zinc. It is possible that a bronze powder pigment is present. Bronze powders are made of copper-zinc (brass) or copper-tin (bronze) alloys (Gettens 1942, 100). It is also possible that the zinc is present as zinc white and the copper as azurite, malachite, and/or verdigris.
It is possible that the background of the WUDPAC tintype is a modification to at least one recipe outlined in Wall’s manual; adding a red lake to Prussian blue, ochre, “black,” and lead white (Table 2). While several pigments have been identified, and it is possible that more are present, it does not appear that a “body color” by Ayre’s definition was used. This description states that “all the colors used” would be mixed with Chinese (zinc) white (Ayres 1878, 110). Strictly speaking, if all of the colors that were used to paint the figure were also used for the background then chrome yellow and vermilion would have to be present since they were found in the gold jewelry and pink lips, respectively. Thus far, analysis has not detected the presence of chrome or mercury in the background area of this plate. In addition, zinc was not detected indicating that zinc white was not used.
Gold Jewelry
The gold jewelry in the WUDPAC tintype appears to have been painted with a very different technique compared to both of the NMAH tintypes. Raman spectroscopy detected chrome yellow and lead white in areas of gold jewelry on the WUDPAC tintype. XRF also detected mercury which indicates the likely use of vermilion. It is possible that more pigments are present. A limited number of sample sites were analyzed but this area of paint is very detailed with many layers of different paints present. To give the appearance of gold, Wall suggests applying one color for the highlights, another for the reflected highlights, another for the local color and yet another for the shadows (Wall [1861] 2009, 84). It appears that the WUDPAC gold jewelry was painted following these suggestions.
The NMAH tintypes demonstrate another approach to the painting of gold jewelry. XRF detected copper and zinc on both plates. This most likely indicates the use of bronze powders. In both cases, the bronze powder is applied to create a fairly flat even field of color. With this approach, the colorist was relying on the metallic sheen of the bronze powder to create the illusion of gold. This was also a common 19th-century approach; however many photographers boasted that they used real gold in their paints (Ferguson 2008, 17). Zinc white may also be present on both plates. The detection of lead, iron, and manganese on NMAH (75.17.957.2) suggests that lead white and iron oxides may also be present.
White Lace
Raman spectroscopy identified lead white in areas of white lace on the WUDPAC and NMAH (6912A) tintypes. Zinc was also detected by XRF on the latter suggesting the presence of zinc white as well. The XRF spectrum for the white lace of NMAH (75.17.957.2) shows peaks for both lead and zinc indicating that lead white and zinc white are likely present. The bright yellow autofluorescence of this area supports the presence of zinc white.
Pink Lips
Vermilion was detected in the pink lips of the WUDPAC tintype using XRF and Raman spectroscopy. The presence of lead, detected by XRF, in the lips of the WUDPAC tintype and NMAH (75.17.957.2) suggests the presence of lead white. The use of vermilion or carmine to color lips is frequently referred to in the literature. Since this is an organic lake pigment, undetectable by XRF and difficult to detect with Raman, it may also be present on the NMAH tintypes but undetected in this study. It is also possible that iron oxides are present in all three cases.
Dark Hair
XRF analysis of the dark hair on the WUDPAC tintype and NMAH (75.17.957.2) suggests the presence of iron oxides and lead white. A calcium peak on the WUDPAC spectrum indicates that chalk may also be present as a filler. Ayres suggests using sepia for coloring dark brown hair (Ayres 1878, 73). Unfortunately, this is an organic colorant which cannot readily be detected by the analytical techniques used in this study. Organic dyes such as madder or Bistre ink could also be present but were not detected.
Lacquer
Cross-section microscopy and UV illumination showed that the lacquer layer of the WUDPAC tintype has a bright orange autofluorescence characteristic of shellac. FTIR analysis also suggested the presence of shellac. The GC-MS spectrum did not pick up peaks characteristic for this material. However, the sample was very small and as a result the signal was weak. It is possible that shellac or other natural resins are present but were not detected. SEM-EDS mapping showed the presence of lead and manganese in areas of a few discrete particles within the lacquer layer. If this were an oil-based (japanned) lacquer layer, lead and manganese could have been used as driers. However it is also possible that these particles were actually on the surface of the sample and were transferred from the paint layer where there was a large lead presence.
This technical study has provided information regarding the manufacture of a select group of whole-plate hand-painted tintypes from the WUDPAC and NMAH collections. An oil binder was identified as were several pigments including; carbon black, umber, lead white, chalk, Prussian blue, chrome yellow, vermilion, and a red lake. All of the materials identified (or suggested) are consistent with known 19th-century hand-coloring materials. Furthermore, some evidence suggests that the coloring of these tintypes follows instructions outlined in 19th-century photography manuals. However, examination and analysis revealed several variations in painting materials and application. This suggests that the hand-coloring of 19th-century portraits was not necessarily as formulaic as the manuals may lead one to believe.
The opaque purplish background paint seen on all of the tintypes chosen for this study appears to be quite ubiquitous. This study showed that the backgrounds on the tintypes examined were consistently unvarnished and in some cases they may have also been intentionally textured, possibly to further heighten their matte appearance. The 19th-century hand coloring manuals consulted suggest that this style of background was predominately used to cover the distractions and defects of the original photographic portrait as well as any evidence of a copy stand used to reproduce the portrait as a whole plate tintype. Several different recipes were identified in the manuals for neutral opaque backgrounds. The analysis conducted also suggests that there were many different pigment combinations which could be used to create a similar looking background paint.
Whole plate hand-painted tintypes may have been commercially produced and targeted at the middle-class, but the results are far from ordinary. This format of 19th-century photography filled a distinct niche of commercial photography and provided the public with colored photographic portraits in a time when photographic science could not do so alone.
I would like to extend my gratitude to all those who assisted with this technical study. This work would not have been possible without the dedicated guidance and assistance of the staff at Winterthur Museum’s Scientific Research and Analysis Laboratory (SRAL). Thank you to Dr. Joseph Weber (Associate Professor, University of Delaware) for overseeing the study and to Dr. Jennifer Mass (Senior Scientist, Winterthur Museum), Catherine Matsen (Associate Scientist, Winterthur Museum) and Dr. Chris Petersen (Consulting Scientist, Winterthur Museum) for providing additional support obtaining and interpreting data. Melanie Gifford (Research Conservator, National Gallery of Art) was extremely helpful in analyzing and interpreting the pigment sample with PLM. I would also like to thank Debbie Hess Norris (Program Chair, WUDPAC), Jae Gutierrez (Assistant Professor, WUDPAC), and Barbara Lemmen (Adjunct Assistant Professor, WUDPAC) for suggesting this topic and allowing the tintype from the WUDPAC study collection to be included and sampled. Finally, I am sincerely appreciative of Richard Barden (Manager of Preservation Services, NMAH) and Michelle Delaney (Curator, Photographic History Collection, NMAH) for allowing the NMAH tintypes to be included in this study and for making their expedited loan to Winterthur Museum possible. The loan would not have been possible without the help of the Registrars at NMAH and Winterthur Museum especially Margaret Grandine (Registrar, NMAH) and Paula De Stefano (Registrar, Winterthur Museum). I sincerely thank everyone for all of their assistance and words of encouragement.
Ayres, G.B. 1878. How to paint photographs in watercolors and in oil. New York: Appleton.
Buck, S. and P. Olley. 2008. Unpublished presentation notes and handouts for ARTC 617: Cross-section microscopy of painted surfaces. Winterthur/UD Program in Art Conservation.
Burns, S.B. 1995. Forgotten marriage: The painted tintype & the decorative frame 1860-1910. New York: The Burns Press.
Carlebach, M.L. 2002. Working stiffs: Occupational portraits in the age of the tintype. Washington D.C.: Smithsonian Institution Press.
Eastaugh, N. ed. 2008. Pigment compendium: A dictionary and optical microscopy of historical pigments. Butterworth-Heinemann.
Estabrooke, E.M. [1872] 1972. The ferrotype and how to make it. Reprint, Hastings-on-Hudson: Morgan & Morgan.
Ferguson, S.H. 2008. In living color: Process and materials of the hand-colored daguerreotype. The Daguerreian Annual: 13-18.
Gettens, R.J. and G.L. Stout. [1942] 1966. Painting materials: A short encyclopedia. Reprint, New York: Dover.
Gihon, John L. 1878. The photographic colorists’ guide. Philadelphia: E. L. Wilson.
Henisch, H.K. and B.A. Henisch. 1996. The painted photograph 1839-1914: Origins, techniques, aspirations. University Park: The Pennsylvania State University Press.
Kashner, S. ed. 2008. America and the tintype. New York: International Center of Photography.
Rinhart, F., M. Rinhart, and R. Wagner. 1999. The American tintype. Columbus: The Ohio State University Press.
Schimmelman, J.S. 2007. The tintype in America 1856-1880. Philadelphia: American Philosophical Society.
Simons, Montgomery P. 1857. Plain instructions for colouring photographs in watercolors and India ink. Philadelphia: T. K. & P. G. Collins.
Simpson-Grant, M. 2000. The use of ultraviolet induced visible-fluorescence in the examination of museum objects, part II. Conserve O Gram 1/10. Washington D.C.: National Park Service.
Swan, A. 1989. French methods and materials for coloring daguerreotypes. In French daguerreotypes. J.E. Buerger. Chicago: The University of Chicago Press. 150-163.
Towler, J. [1864] 1969. The Silver Sunbeam. Reprint, New York: Morgan & Morgan.
Trask, A.K.P. [1872] 1973. Practical ferrotyper. In The collodion process and the ferrotype: Three accounts, 1854-1872. ed. R.A. Sobieszek. Reprint, New York: Arno Press.
University College of London. 2009. Raman spectroscopic library of natural and organic pigments. http://www.chem.ucl.ac.uk/resources/raman/index.html
Wall, A.H. [1861] 2009. A manual of artistic colouring, as applied to photographs: A practical guide to artists and photographers. Reprint, Danvers: General Books.
ALISHA CHIPMAN
The National Gallery of Art, Washington D.C.
DR. JOSEPH N. WEBER AND DR. JENNIFER L. MASS
Winterthur/University of Delaware Program in Art Conservation
Papers presented in Topics in Photographic Preservation, Volume Fourteen have not undergone a formal process of peer review.