Topics in Photographic Preservation 2011, Volume 14, Article 41 (pp. 263-281)
The orotone photographic process utilizes a positive image on glass that is subsequently coated with bronze powders or backed with reflective materials. Materials used in the creation of four vintage orotones by different producers and dating from the early 20th-century are characterized in this study. No previous study has been performed to characterize all the materials present in an orotone. One study was performed in 1986 to determine whether gold had been used as a backing material (Rempel 1986). In this study a technical analysis was performed using ultraviolet fluorescence, x-ray fluorescence (XRF), Fourier-transform infrared spectroscopy (FTIR), and scanning electron microscopy coupled with energy dispersive x-ray spectroscopy (SEM-EDS). The presence of gelatin and/or collodion is suggested as the photographic image emulsions used to create the orotones. Bronze powders containing copper and zinc were used in a binder to back each of the images studied. The binder in each case appears to contain cellulose nitrate.
The orotone process enjoyed limited popularity during the late 19th-century through the 1940’s. The objects were produced as art objects, and more commercially as souvenirs for sale at tourist destinations (Osterman). An orotone, (also known as Doretype, goldtone or Curt-tone) is a positive photographic image on glass. This study focuses on the analysis of four orotone photographs by different makers. Technical analysis was used to provide insight into the method of manufacture and identification of the materials present in the image, backing, and coating layers that comprise the orotones. This enhanced understanding of materials and historical processing may help determine appropriate materials and methods for the conservation and preservation of these objects.
Analyzed objects
Four orotone photographs were analyzed. The orotones were in very good condition, and were housed in their original frames and paper backing materials (see illustration 1).
Historical processing
Understanding historical processing techniques and materials provides valuable information about what materials are present in orotone photographs. In the late 19th- and early 20th-centuries gelatin or collodion emulsions were widely used to bind light sensitive materials to glass to create negative or positive images. Gelatin is the product of carefully refined and processed animal skin, and collodion is made from dissolved, nitrated cotton in alcohol and ether. The use of collodion as a binder was introduced in the early 1850’s, and was largely superseded by the use of gelatin on glass plates in the late 19th and early 20th-centuries.
Collodion was used in wet-plate processing, where light-sensitive emulsion had to be applied, exposed and developed before completely drying and becoming insoluble. Pre-sensitized glass plates using a gelatin binder were called dry plates. They gained popularity with professional and amateur photographers in the 1880’s and 1890’s. The gelatin dry plate did not require processing while still wet, greatly simplifying the photographic process. Dry plates were sold commercially and were widely used during and after this era, eventually being largely replaced by film support negatives in the early 1900’s. It is likely that both collodion and gelatin emulsions were used to create orotones because the dates that these images were made extend through this transitional time period.
Only one specific reference could be located for the creation of orotones. It was an article in the April 1922 edition of Studio Light, published by the Eastman Kodak Co. The directions propose creating a thin positive image on glass by exposing the printed image fully from a negative, but limiting the degree of development. The positive image formed is then bleached until the black of the shadow areas disappears. The bleaching solution is composed of potassium ferricyanide, potassium bromide and water. The image is then redeveloped in a “redeveloping solution” that is presumed to be a modified silver bath (in sodium sulfide/water) until the original detail returns, and rinsed in a hardening bath composed of sodium sulfite, acetic acid and alum in water. The redeveloped positive is washed, dried, and ready for backing.
For this backing a “… very fine, natural gold-color bronze that will give a smooth surface…(and it)…must be combined with a liquid that will not affect the silver deposit or the gelatine and that is as nearly colorless as possible,” (Studio Light 1922, 8). Eastman Lantern Slide Varnish is recommended in the article because it dries in 30 minutes and remains colorless. The bronze varnish solution is to be brushed on and should be thin enough to flow together without leaving brush marks.
Edward Curtis was a photographer famous for his work capturing images of Native Americans. He is credited with the invention and sometimes employed the orotone process. He felt that the process yielded superior images wherein “…transparency is retained and they are … full of life and sparkle,” (Davis 1985, 66). The application of the reflective metallic backing was described by one of his former employees as being applied by “tipping and rolling an amount of the gold liquid over the surface until it was evenly covered”, and that the process caused the building to overwhelmingly smell of bananas (Davis 1985, 66). Such accounts provide clues to the ingredients and methods used to create orotone images. Banana oil (amyl acetate) was apparently used in the process by Curtis, perhaps as a flowing or wetting agent.
Lantern slides
Viewing lantern slides was a popular form of entertainment in the late 19th-century. The slide images were projected onto a wall or screen in a manner analogous to modern slide projectors. Lantern slides are similar to orotones, possessing a positive image on a glass support, but without the bronze coating. The images could be created by drawing or painting directly on the glass support, or through photographic methods using collodion or gelatin. By 1900 many varieties of photographic chemicals, emulsions, and coatings could be purchased “ready-made”. References to how lantern slides were produced provide valuable additional information for understanding orotone image processing (Fraprie 1918).
Photographic emulsions
Collodion consists of gun-cotton (nitrated cellulose) dissolved in ether or ether/alcohol solutions. The collodion photographic process was introduced in 1851 using potassium iodide in collodion poured onto a glass plate. Before the ether completely evaporates the plate is plunged into a bath of silver nitrate to sensitize it. While still slightly wet or tacky the plate is placed into a plateholder, exposed to light in a camera, and developed. This is why the process is referred to as the “wet plate process”. Wet plate (collodion) photographic methods sometimes employ a preliminary albumen or gelatin “subbing” layer before applying collodion to the glass plate. The subbing layer coats the glass and helps the collodion adhere more completely to the plate. Once processed, plates that appear cloudy can be cleared using a solution of iodine and potassium iodide. Toning agents such as platinum or gold chloride can be added to alter the tonality of the image and to make it more permanent.
Gelatin “dry plates’ were often used for lantern slides. Dry plates require a minimal amount of exposure time and preparation, making them popular among photographers. A method for making gelatin plates at home was to combine:
110 grains gelatin
62 grains potassium bromide
0.5 ounces 10% salicylic acid in alcohol
2 ounces water
The solution is heated slightly until the gelatin swells and goes into solution.
Separately combine:
77 grains silver nitrate
2 ounces water
And crush the crystals until they disappear. To this add ammonia, drop by drop, until the solution becomes dark, and then clears. Heat the solutions for 15 minutes, remove from heat and mix the silver solution with the gelatin mixture. Once the two mixtures have been combined the solution must be kept in darkroom conditions.
The solution is allowed to cool to room temperature and set into a gel. This is washed in water to remove water-soluble potassium nitrate, leaving a gelatin emulsion containing light-sensitive silver bromide. The emulsion is then filtered through cloth in a succession of water baths. Finally the emulsion is reheated, filtered and coated onto clean glass that is placed horizontally until the gelatin sets (Hepworth 1978, 146-147). The dry plate was now ready for conventional exposure and development to create the image. After the image was created it would be varnished and/or encapsulated by attaching a second sheet of glass behind the image. Varnishing was often not performed on lantern slides because of problems with heat during projection (Osterman).
Backing materials
The use of bronze powders for gilding purposes was common in the late 19th- and early 20th century. Bronze powders were sometimes applied to glass to create lettering and background color on shop windows. Vintage reference works contain recipes for creating solutions containing bronze powders, and for methods of applying them. One such recipe for the so-called “banana solution” (named for its odor) contains amyl acetate, acetone and benzine in equal amounts, with just enough pyroxyline dissolved therein to give it body. Pyroxyline is an archaic name for nitrocellulose or collodion. The thin solution was brushed on and would settle flat before drying. The collodion was intended to leave a thin protective covering on the bronze, preventing deterioration from contact with the air (Hiscox and Sloane 1944). Another recipe also recommends the use of “banana liquid”, calling for collodion dissolved in amyl acetate. A possible, partial substitution for some portion of the more expensive amyl acetate could be made using benzine, and gum can be used in place of some of the collodion, but substituted coatings were not as durable as the original banana liquid (Hoff 1905).
Two vintage recipes for collodion varnish consists of:
1) 1.5 pounds gun cotton (dry)
10 pounds acetone
dissolve the gun cotton in the acetone, then add
20 pounds amyl acetate
10 pounds benzine
allow to settle and pour off the clear solution or filter if desired
2) 1 gallon amyl acetate
1 gallon benzol
10 ounces gun cotton (Hoff, 1905, 170)
These varnishes could be made more flexible with the addition of 4 ounces Castor oil per gallon of varnish. Wax could also be added to produce a less glossy varnish.
Visible light
The image materials of the orotones were examined in visible light using a 10x magnifying loupe.
UV Fluorescence
Ultraviolet examination was performed on the recto and verso of each orotone after they were removed from their frames. A handheld UVP Model UVL-56 Blak-Ray lamp was used to examine the objects using shortwave and longwave UV light. Observed fluorescence was recorded in handwritten notes and compared to known samples present in the examination area. Attempts were also made to photograph the objects digitally.
X-Ray Fluorescence (XRF)
XRF analysis of each orotone was performed using an ARTAX Micro XRF spectrometer with molybdenum and tungsten tube sources with the following settings:
Voltage: 50 kV
Current: 600 ΜA
Acquisition time: 300 seconds
Additional measurements were taken using the molybdenum source for a longer run to verify the results of spectra generated for the Cypress orotone with the following settings:
Voltage: 50 kV
Current: 600 ΜA
Acquisition time: 900 seconds
No sampling was required because an open-architecture instrument was used. The orotones were placed individually, usually face down, in front of the x-ray source on an ethafoam support.
Fourier-Transform Infrared Spectroscopy (FTIR)
FTIR analysis was performed on samples from each orotone using a Nicolet Magna 560 Infrared Spectrometer in conjunction with a Nic-plan microscope with a 4 cm-1 resolution and 4000-650 cm-1 range (number of scans: 120). Samples were collected from the bottom edge of the image faces on the Half Dome and Birches images, and on the perpendicular edges of the Cypress and Le Sesne images. Sampling was restricted to areas the frame rabbet will cover when the objects are re-framed. A sharp scalpel that had been carefully cleaned with methanol was used to collect the samples under magnification. The samples were transferred to a diamond cell support and flattened using a steel roller tool, then placed on the stage of the FTIR microscope. The resulting spectra were interpreted using Nicolet 6.0 OMNIC software and spectral reference libraries on the Winterthur Museum’s Scientific Research Analytical Laboratory (SRAL) computer. Some spectra were baseline corrected before being compared to computer reference spectra for potential matches.
An extraction was performed on one sample from the Half Dome orotone using acetone. The extraction was done with the sample placed on a microscope slide. One drop was applied at a time and the sample was allowed to dry between applications. A total of 5 drops were applied and a hazy film was observed on the microscope slide. This residue was collected by scraping the slide with a clean scalpel, transferred to a diamond disk, and examined with the FTIR microscope.
Scanning Electron Microscopy (SEM)
A Topcan ABT-60 scanning electron microscope adjusted with a gold reference standard was used to analyze the elemental composition of the orotones using energy-dispersive x-ray spectroscopy (SEM-EDS). Samples were placed on a carbon stub and transferred to the stage (height 22 mm) of the microscope before pulling a vacuum in the sample chamber. Using an EVEX pulse processor and software, the samples were rotated and focused in the x- and y-planes to select sites for analysis. An EDAX 9900 detector was used to collect data at voltages of 15-20 kV. Back-scattered electron imaging was used to examine the morphology and structure of the sample’s components. The major elements found using SEM-EDS were mapped to establish their distribution on the samples. One sample was collected from the lower edge of the Half Dome image face, and analyzed to investigate the stratigraphy of the sample. This sample was cast in Bio-Plastic Casting plastic catalyzed with methyl ethyl ketone peroxide hardener. This resin cube was cured under a lamp for one hour and allowed to fully cure at room temperature for 48 hours. The cured plastic cube was sanded on one face to expose the sample and subsequently transferred to the SEM stage. Particle size ranges were measured using Evex Nanoanalysis software.
Visible Light
Examination under 10X magnification revealed that the Birches, Le Sesne, and Half Dome orotones were continuous-toned, with the gradual increase in image density associated with photographic processes. Density areas on the Cypress orotone were formed by a series of dots of differing densities.
Ultraviolet Fluorescence
Examination under ultraviolet light revealed subtle fluorescent differences visible on the verso of the orotones. Only minimal fluorescence was observed, and was more noticeable under longwave radiation. Reflection from the glass surface made examination of the recto face unsuccessful for distinguishing fluorescent information from the objects.

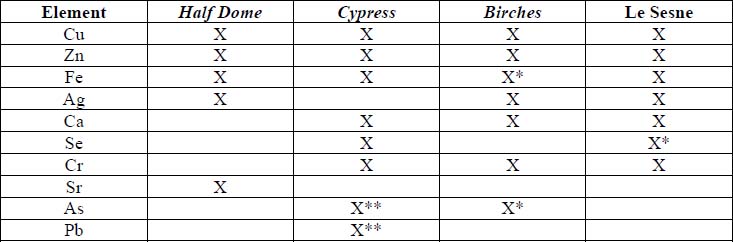
X-Ray Fluorescence
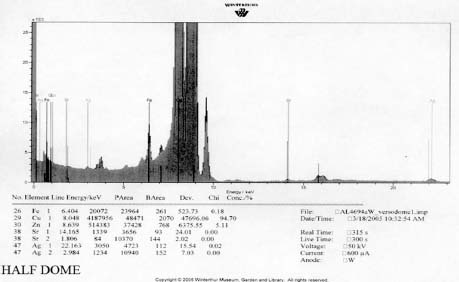
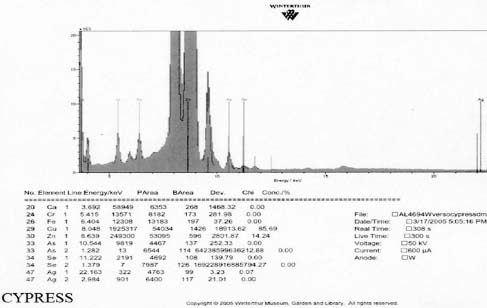
XRF analysis of the orotones was performed at random sites on each of the objects using a 300 second run time. The resulting spectra suggest the presence of copper, zinc, and iron on each of the objects. The presence of small amounts of chromium was suggested on three of the orotones. Multiple runs were made on the Cypress orotone attempting to confirm the presence or lack of a significant peak for silver. One additional run was made on this object with a 900 second run time. Additional elements suggested as being present by the data include potassium, arsenic, strontium and barium.
Summary of XRF Examination
Mo and W sources, 50kV, 300s
*denotes trace amounts possibly detected
** because the peaks for As and Pb are very close on the spectra generated, it is uncertain whether one or both these elements are suggested as present in the Cypress orotone. This was not resolved using SEM-EDS.
Fourier Transform Infrared Spectroscopy
FTIR analysis was performed on samples collected from the image face of the Birches and Half Dome orotones. Reference library software was used to provide reasonable matches for the spectra generated from these samples (see appendix 1-FTIR). A strong match was found for proteinaceous materials in each case. Reference spectra for aged gelatin (aged 5+ years, Salimnejad, PMA, tran) were compared with that of the samples, and there were strong similarities, with typical amide peaks matching near 1650 cm-1, and 1550 cm-1. Other materials were also thought to be present. The spectrum for aged gelatin was subtracted from the original sample spectra and new spectra generated. A new search for a match for this (sample minus gelatin) subtraction spectrum was found using the computer reference libraries. A second component suggested as present in each spectra was cellulose nitrate (age 63.5 yrs, Gettens, pnl 71.85, PMA, trn). Again this matching spectrum was subtracted from the previous spectra and a match generated for further comparison. The remaining spectrum provided a potential match for metal stearates.
An extraction was performed on a sample from the Half Dome orotone using acetone. The residue that had been solubilized in acetone was analyzed and the spectrum generated was compared to library reference spectra.
Samples from the side edges of Le Sesne and Cypress images were also analyzed. The Cypress sample also had peaks consistent for the presence of cellulose nitrate and possibly for proteinaceous materials. When spectra for those materials were subtracted, a close match for metal stearate was suggested by the reference libraries. The Le Sesne sample presented minor or almost no peaks suggesting the presence of proteins. Its spectrum was compared to reference spectra and closely matched cellulose nitrate. When the spectrum for cellulose nitrate was subtracted from the sample spectrum, the best match was again for metal stearates.
Scanning Electron Microscopy
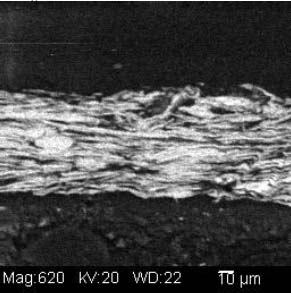
SEM-EDS spectra examined suggest the presence of copper, zinc, silver, and sulfur. To examine the morphology and distribution of these elemental components several magnified images were generated using backscattered electron imaging. Locations on the samples were chosen that displayed a variation of brightness and morphology. The metallic particles were observed under magnification as very thin, or flat, and having clastic shapes with irregular edges. Measurement of particle size was possible using computer software displaying a scale bar with the magnified imagery. Using computer software the particle sizes observed in a representative sample measured 22-57 microns along their longest observed axis (see illustration 2). One sample that had been cast in a resin cube was examined in cross-section. The metallic particles were observed to be tightly aligned in a parallel direction (see illustration 3).
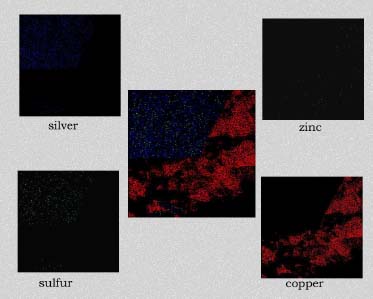
Image mapping using ED-XRF also provided graphic representation of the distribution of the elements having major peaks in the spectra (see illustration 4). Silver was observed on the smooth face of the sample with small amounts of sulfur dispersed in the silver. Copper particles with small amounts of zinc were observed as the predominant materials contained in the irregularly shaped surface.
Image material
XRF analysis suggested the presence of silver as the image material, with the possibility of selenium or sulfur as toning agents on the Half Dome, Birches, and Le Sesne orotones (see appendix 1-XRF). The identification of the image material on the Cypress orotone was not resolved by analysis. No significant peak for silver or other metals typically associated with photographic processes was observed using XRF despite repeated measurement of different areas of maximum density on this orotone. Additional testing using SEM-EDS was not performed due to the time constraints of this study and to minimize sampling from the image surface. Additional materials suggested by using XRF for a 900 second run time included potassium, arsenic, strontium and barium. These materials, along with iron and calcium, are all materials likely to be associated with the glass support.
Under magnification the density areas of the Cypress orotone exhibit a dot pattern consistent with that of half tone prints. The dot pattern observed is due to a screen that breaks the image up into discrete units of density for printing. It is unclear whether the image was created photographically, photo-mechanically, or through some other technique such as photosilk-screening. Photo-silk-screening can be used to transfer an image to glass either directly, via special glass paints, or indirectly with decals (Wade 1978).
Image emulsion
The presence of proteins was suggested by FTIR analysis on the Half Dome, Birches, and to a lesser extent Cypress orotones (see appendix 2-FTIR). It is possible that the presence of proteinaceous materials is due to the use of a gelatin dry plate to create the images. This is especially likely in the later years associated with the process, 1920-1950 when the collodion wet plate had been virtually eliminated from use.
FTIR analysis suggests that collodion is present and may be the image emulsion for the Le Sesne image. Sampling was limited to a side edge rather than the image face of this orotone, so it may have been made using other emulsion materials as well. This image has a different appearance on the backing layer. There are small, raised ridges of thicker layers present suggesting that some part of the image was poured on and worked by hand, rather than being applied as a uniform coating or possibly sprayed surface.
Backing materials
Bronze powders are suggested as the backing materials present on each orotone by XRF spectroscopy (see appendix 1-XRF). No significant peaks were observed to suggest that gold was present as is sometimes suggested in the descriptions of orotones. The composition of the powders appears to be primarily Cu and Zn in varying proportions adjusted to suit the preference of the maker to achieve the desired color. A 10% zinc alloy appears bronze while a 15% alloy has a golden appearance. The materials observed are consistent with the results of XRF research published by Rempel (1986). No attempt was made to quantitatively characterize the percentages of the components of the alloys. Quantitative analysis of the alloy would be difficult to establish without having standards with the same matrix as the analyte.
The bronze particles appear to have metal stearates present. These materials could be present as residues from grinding lubricants used in manufacturing the powders or as a thixotropic agent to adjust the binder’s viscosity. Metal stearates consist of long hydrocarbon chains with metallic ions on one end, and are usually derived from beef fat solids. A 1905 source states that the powdered metals are “… ground and heated with a little oil, grease, wax, or paraffine…” (Hoff 1905 p. 79). This description of the powder-making process may account for why metal stearates are present.
The bronze particles that were examined for size measured between 22-57 microns along their longer observable axis using SEM. This size is consistent with particle sizes listed in sales literature from the Leo Uhlfelder Co. (LUCO), a modern supplier of bronze powders. Size and shape of particles affect the optical characteristics of the powders.
Binder for powders
All samples examined with FTIR suggest the presence of cellulose nitrate (see appendix 2-FTIR). It is likely that collodion was used in the binder for the metallic powders, as it appears as an ingredient in the historical recipes previously cited. The presence of cellulose nitrate may explain why the bronze powders do not show significant signs of deterioration or adversely impact the silver image material. Image fading due to contact with the zinc component of bronze powders is a common problem on photographic prints. When particles from bronze inks come into contact with silver image materials the silver is oxidized, which results in a faded spot. Storage in elevated relative humidity conditions accelerates such deterioration mechanisms (Reilly 1986).
The presence of metal stearates in all samples was also suggested by FTIR analysis. Metal stearates may be present in the binder as a stabilizer to keep the metallic particles in solution. Stabilizers regulate consistency, and 1-2% aluminum stearate is typically added to paints (Saitzyk 1987). Aluminum stearate can be “used in small amounts to prevent separation of pigments from binding oils, it is also added to varnishes to produce flat, non-glossy coatings.” (Gettens and Stout 1966, p.93). By the early to mid 1920s, aluminum stearate was available for commercial use. Aluminum stearate is often included in artist’s paints without being specifically listed as a component (Tumosa 2001).
Old recipes call for the use of amyl acetate, the active chemical ingredient in banana oil, in the coating process. Amyl acetate probably acts as a wetting agent, with molecules that have one polar and one hydrophobic end. As a solvent it may have the ability to diminish interactions between the particles while maintaining a suitable viscosity to allow the particles to lie flat and orient themselves in a parallel manner before drying.
The analysis of samples from four orotones has led to an enhanced understanding of the materials used in their manufacture. Proteinaceous materials found on two examples suggest that such images were often made using gelatin dry plates. Three of the images were produced photographically and have continuous density tones. The presence of silver was suggested and probably forms the image material for this group of orotones. The silver image material may have been toned. Protein was not detected on samples taken from the edges of the Le Sesne orotone. Collodion (cellulose nitrate) was commonly used as emulsion for photographic image materials in the late 19th-century and was suggested as present on the Le Sesne image through FTIR analysis. While proteins were also suggested for the Cypress image, no indication of silver or other metallic image material was observed. This may be due to the absence, or low amount of image material present, or due to non-photographic means of image production. This image has a distinct dot pattern that can be observed under 10X magnification consistent with half-tone printing processes. Inks containing elements for color such as carbon may be the final image material on this image. Further testing and sampling from image areas would be necessary to determine the accuracy of this hypothesis. Such sampling is aesthetically intrusive and destructive to the object, and as such was not undertaken or recommended. Bronze powders composed primarily of copper and zinc are believed to be the backing materials on the image and glass support on each orotone. FTIR analysis of the four orotones suggests that cellulose nitrate was used as a binder for the powders. The presence of metal stearates was also suggested in every sample analyzed.
The presence of bronze powder materials in binder was observed on side edges of the glass support on all orotones examined (see illustration 5). This original material could be used in small repairs of areas of loss due to scratches, abrasion, or contact with backing and housing materials. Bronze powders used for repairs from other sources should be coated in a suitable material or adhesive to prevent the potential interaction with existing metallic materials present. Aquasol may be a suitable adhesive for applying bronze powders that will remain reversible in water (Pouliout). Additional orotone images should be examined to further establish and confirm the historical methods and materials of manufacture. A variety of images representing different makers and time periods should be sought to provide the widest range of information. Broken or damaged images with minimal value would make excellent candidates for additional sampling and analysis.
I would like to thank the following people for their assistance with this technical study: Barbara Lemmen, for providing the Birches orotone for technical analysis; Debra Hess Norris, for guidance and suggestions in creating the technical study; Dr. Jennifer Mass, for performing SEM analysis, interpretation of results, and assistance during the study; Janice Carlson, for assistance with FTIR procedures and analysis of results; Catherine Matsen, for help with operation of the XRF instrument and interpretation of its data; Dr. Joseph Weber, for assistance with the XRF instrument, FTIR spectra, and numerous, helpful suggestions and interpretation of results while supervising the study; Mark Anderson, for providing vital source materials; Bruno Pouliot and Richard Wolbers for their useful comments and insights about adhesives, solvents, and bronze powders.
Davis, B. 1985. Edward S. Curtis, life and times of a shadow catcher. San Francisco: Chronicle Books.
Fraprie, F. 1918. How to make lantern slides. Boston, Mass.: American Photographic Publishing Co.
Gettens and Stout 1966. Painting materials; a short encyclopedia. New York: Dover Publications. p. 93
Hepworth, T. C. 1978. The book of the lantern. NY: Arno Press.
Hiscox, G. D. and T. O. Sloan. 1944. Bronzing solutions for paints. Fortunes in formulas for home, farm, and workshop. New York: Books, Inc. p 489-490.
Hoff, J. N. 1905. Bronzing liquids. Paint and varnish facts and formulae. Newark, New Jersey: Central Publishing Company.
Osterman, M. May 2005. Personal communication about orotone processing. George Easman House, Rochester, NY.
Pouliot, B. 2005. Personal communication. Winterthur Museum, Winterthur, Delaware.
Reilly, J. M. 1986. Care and identification of 19-th century photographic prints. Rochester, New York: Silver Pixel Press.
Rempel, S. 1986. Energy dispersive x-ray fluorescence applications in the examination of historic photographic artifacts. The paper conservator. 12:80-85.
Saitzyk, S. 1998. The definitive guide to artists’ materials. Melbourne, Australia: Watson-Guptill.
Studio Light. 1922. A beautiful process that is still in demand. 14(2), (April): 6-10. Rochester, NY: Eastman Kodak Co.
Tumosa, C. S. 2001. A brief history of aluminum stearate as a component of paint. Western association for art conservation newsletter 23(3) (September):1-3.
Wade, K. E. 1978. Alternative photographic processes. Dobbs Ferry, New York: Morgan and Morgan. p. 47-48.
Wolbers, R. December 2004. Personal communication. Winterthur Museum, Winterthur, Delaware
Baldwin, T. S. 1903. Picture making for fun and profit. Chicago Illinois: Frederick J. Drake & Co. p. 231-243
Brown, B. 2004. Personal communication, Humanities Research Center, University of Texas, Austin, TX.
Carlson, J., K. Duffy, and A. Tagle. 2003. Course manual, Analytical techniques in conservation. Williamstown Art Conservation Center, July 6-11th, 2003.
Davis, B. 1985. Edward S. Curtis, life and times of a shadow catcher. San Francisco: Chronicle books.
Gettens and Stout 1966. Painting materials; a short encyclopedia. New York: Dover Publications. P. 93
Haist, G. M. 1979. “Chemical Toning”. Modern photographic processing. Okemos, MI: Haist Press, p 107-145.
Hepworth, T. C. 1978. The book of the lantern. NY: Arno Press.
Hiscox, G. D. and T. O. Sloan. 1944. Bronzing solutions for paints. Fortunes in formulas for home, farm, and workshop. New York: Books, Inc. p 489-490.
Hoff, J. N. 1905. Bronzing liquids. Paint and varnish facts and formulae. Newark, New Jersey: Central Publishing Company.
How to make lantern slides (no date). Rochester, NY:Eastman Kodak Co.
Metzner, J. 2003. “The technical study of two opaltypes”. Unpublished Winterthur Graduate Program research project.
Munson, D. May 2005. Personal communication about orotone chemical composition. Chicago Albumen Works, Housatonic, Massachusetts.
Nadeau, L. 1997. Encyclopedia of printing, photographic and photomechanical processes. New Brunswick, Canada, published by the author.
Osterman, M. May 2005. Personal communication about orotone processing. George Eastman House, Rochester, NY.
Pringle, A 1898. The Barnet book of photography. London: Elliott & Son, Barnet, Herts.
Penichon, S. December 2004 Personal communication about orotone research. Amon carter Museum, Fort Worth, Texas.
Pouliot, B. 2005. Personal communication. Winterthur Museum, Winterthur, Delaware.
Reilly, J. M. 1986. Care and identification of 19-th century photographic prints. Rochester, New York: Silver Pixel Press.
Rempel, S. 1986. Energy dispersive x-ray fluorescence applications in the examination of historic photographic artifacts. The paper conservator. 12:80-85.
Saitzyk, S. 1998. The definitive guide to artists’ materials. Melbourne, Australia: Watson-Guptill.
Studio Light. 1922. A beautiful process that is still in demand. 14 (2), (April): 6-10. Rochester, NY: Eastman Kodak Co.
Tumosa, C. S. 2001. A brief history of aluminum stearate as a component of paint. Western association for art conservation newsletter 23(3) (September):1-3.
Wade, K. E. 1978. Alternative photographic processes. Dobbs Ferry, New York: Morgan and Morgan. p. 47-48.
Wall, E. J. 1940. Photographic facts and formulas. Boston: American Photographic Publishing Co. p. 265-280
Wolbers, R. December 2004. Personal communication. Winterthur Museum, Winterthur, Delaware.
RICHARD STENMAN
The Better Image
Papers presented in Topics in Photographic Preservation, Volume Fourteen have not undergone a formal process of peer review.

Half Dome

Birches

Cypress

Le Sesne




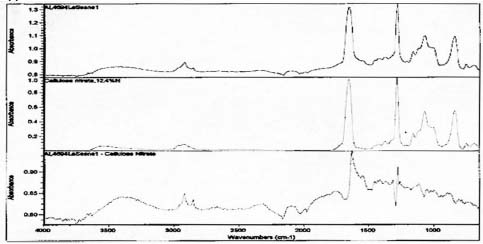
Spectra for Le Sense suggests presence of collodion and the absence of gelatin.
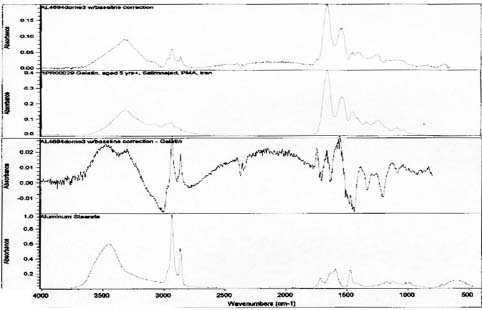
Spectra for Half Dome suggests presence of gelatin and metal stearates.
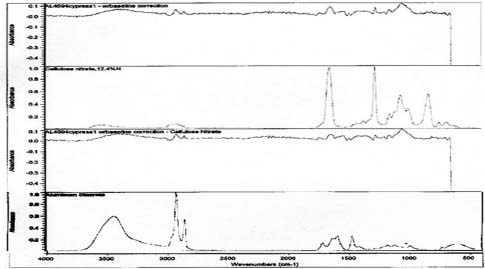
Spectra for Cypress suggests the presence of collodion and metal stearates.
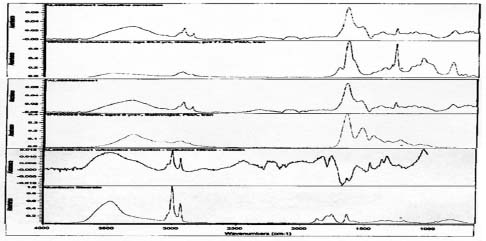
Spectra for Birches suggests presence of gelatin, collodion and metal stearates.